NPs Basic Information
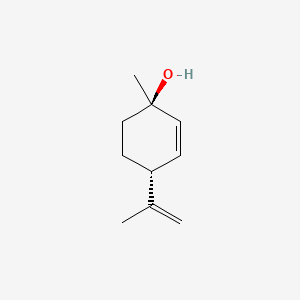
|
Name |
(1r,4r)-1-Methyl-4-(prop-1-en-2-yl)cyclohex-2-en-1-ol
|
| Molecular Formula | C10H16O | |
| IUPAC Name* |
(1R,4R)-1-methyl-4-prop-1-en-2-ylcyclohex-2-en-1-ol
|
|
| SMILES |
CC(=C)[C@@H]1CC[C@@](C=C1)(C)O
|
|
| InChI |
InChI=1S/C10H16O/c1-8(2)9-4-6-10(3,11)7-5-9/h4,6,9,11H,1,5,7H2,2-3H3/t9-,10-/m0/s1
|
|
| InChIKey |
MKPMHJQMNACGDI-UWVGGRQHSA-N
|
|
| Synonyms |
52154-82-2; 7212-40-0; (1r,4r)-1-methyl-4-(prop-1-en-2-yl)cyclohex-2-en-1-ol; (1R,4R)-1-methyl-4-prop-1-en-2-ylcyclohex-2-en-1-ol; (+)-trans-p-Mentha-2,8-dien-1-ol; (1R,4R)-p-Mentha-2,8-dien-1-ol; (1R-TRANS) 1-METHYL-4-(1-METHYLETHENYL)-2-CYCLOHEXENE-1-OL; R1AUQ945JN; 7K859030EU; trans-1-Methyl-4-(1-methylethenyl)-2-cyclohexen-1-ol; (1R,4R)-1-Methyl-4-(prop-1-en-2-yl)cyclohex-2-enol; 4-Isopropenyl-1-methyl-cyclohex-2-en-1-ol, (1R*,4R*), rel-; 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethenyl)-, (1R,4R)-; 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethenyl)-, (1R,4R)-rel-; 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethenyl)-, (1R-trans)-; 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethenyl)-, trans-; (1R,4R)-1-methyl-4-(1-methylethenyl)-2-cyclohexen-1-ol; rel-(1R,4R)-1-Methyl-4-(prop-1-en-2-yl)cyclohex-2-en-1-ol; cis-p-Mentha-2,8-dien-1-ol; UNII-R1AUQ945JN; (Z)-p-Mentha-2,8-dien-1-ol; UNII-7K859030EU; p-Mentha-2,8-dien-1-alpha-ol; EINECS 230-595-4; FEMA No. 4411, trans-(+-)-; (+-)-trans-p-Mentha-2,8-dien-1-ol; 2,8-P-Menthadien-1-ol, trans-(+-)-; cis-2,8-Menthadien-1-ol; SCHEMBL1114908; trans-1-Methyl-4-(1-methylvinyl)cyclohex-2-en-1-ol; cis-p-Mentha-2,8-diene-1-ol; CHEBI:171978; (1R,4R)-1-methyl-4-(1-methylvinyl)-cyclohex-2-ene-1-ol; DTXSID401301235; p-Menth-2,8-dien-1-ol, cis-; p-Mentha-2,8-dien-1-ol, cis-; ZINC5158342; MFCD08460037; AKOS006288261; Mentha-2,8-dien-1-ol, para, cis-; (1R,4R)-p-Mentha-5,8-diene-1-ol; FEMA NO. 4411, TRANS-(+)-; P-MENTHA-2,8-DIEN-1-.ALPHA.-OL; P-MENTHA-2,8-DIEN-1-OL, TRANS-; 2,8-P-MENTHADIEN-1-OL, TRANS-(+)-; P-MENTHA-2,8-DIEN-1-OL, (1R,4R)-; 2,8-P-MENTHADIEN-1-OL, TRANS-(+/-)-; 4-Isopropenyl-1-methyl-2-cyclohexen-1-ol-, cis; P-MENTHA-2,8-DIEN-1-OL, TRANS-(+/-)-; (1R,4R)-4-Isopropenyl-1-methyl-2-cyclohexen-1-ol; 1-Methyl-4-(1-methylethenyl)-2-cyclohexen-1-ol, cis-; 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethenyl), cis; (1R,4R)-4-ISOPROPENYL-1-METHYL-CYCLOHEX-2-EN-1-OL, (E)-; (+/-)-(1R,4R)-4-ISOPROPENYL-1-METHYL-CYCLOHEX-2-EN-1-OL, (E)-
|
|
| CAS | 52154-82-2 | |
| PubChem CID | 111274 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.23 | ALogp: | 2.4 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.573 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.301 | MDCK Permeability: | 0.00002380 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.02 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.942 | Plasma Protein Binding (PPB): | 64.63% |
| Volume Distribution (VD): | 1.435 | Fu: | 44.07% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.139 | CYP1A2-substrate: | 0.826 |
| CYP2C19-inhibitor: | 0.155 | CYP2C19-substrate: | 0.849 |
| CYP2C9-inhibitor: | 0.025 | CYP2C9-substrate: | 0.544 |
| CYP2D6-inhibitor: | 0.013 | CYP2D6-substrate: | 0.489 |
| CYP3A4-inhibitor: | 0.233 | CYP3A4-substrate: | 0.31 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.104 | Half-life (T1/2): | 0.752 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.068 |
| Drug-inuced Liver Injury (DILI): | 0.041 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.041 |
| Skin Sensitization: | 0.061 | Carcinogencity: | 0.152 |
| Eye Corrosion: | 0.927 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.039 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000872 | 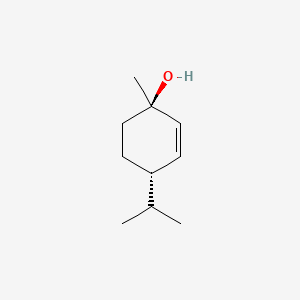 |
0.487 | D07QKN |  |
0.220 | ||
| ENC002264 | 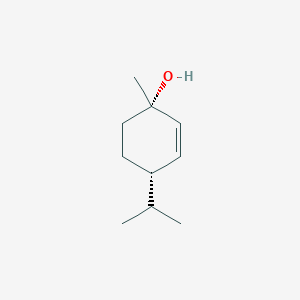 |
0.487 | D0P0HT | 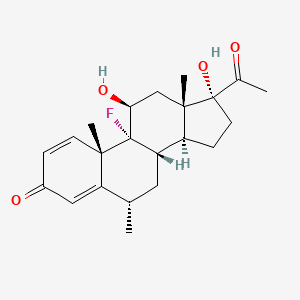 |
0.188 | ||
| ENC002860 | 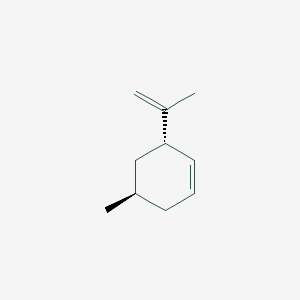 |
0.366 | D0H1QY |  |
0.184 | ||
| ENC001835 | 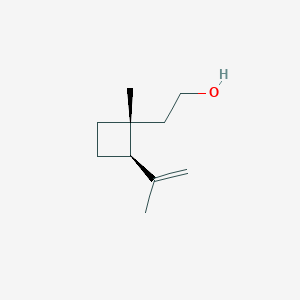 |
0.349 | D04GJN | 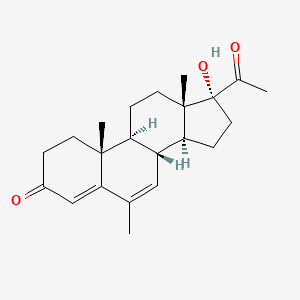 |
0.183 | ||
| ENC005066 |  |
0.276 | D0K7LU |  |
0.182 | ||
| ENC002124 |  |
0.276 | D0O1UZ |  |
0.179 | ||
| ENC002051 |  |
0.276 | D0F1UL |  |
0.177 | ||
| ENC005497 |  |
0.276 | D0IL7L |  |
0.176 | ||
| ENC003085 | 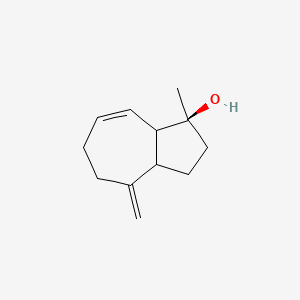 |
0.275 | D06AEO | 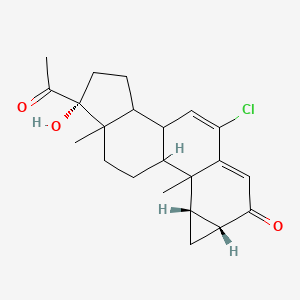 |
0.176 | ||
| ENC002073 | 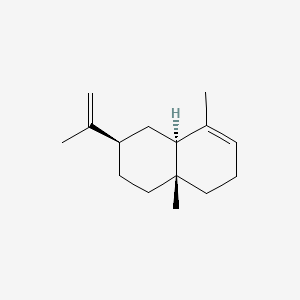 |
0.273 | D0D1SG | 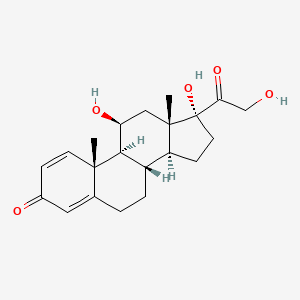 |
0.176 | ||