NPs Basic Information
|
Name |
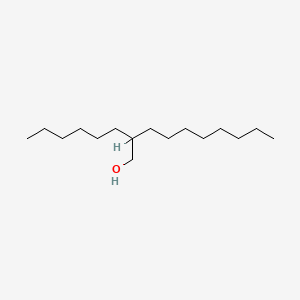
2-Hexyl-1-decanol
|
| Molecular Formula | C16H34O | |
| IUPAC Name* |
2-hexyldecan-1-ol
|
|
| SMILES |
CCCCCCCCC(CCCCCC)CO
|
|
| InChI |
InChI=1S/C16H34O/c1-3-5-7-9-10-12-14-16(15-17)13-11-8-6-4-2/h16-17H,3-15H2,1-2H3
|
|
| InChIKey |
XULHFMYCBKQGEE-UHFFFAOYSA-N
|
|
| Synonyms |
2-Hexyl-1-decanol; 2425-77-6; 2-Hexyldecan-1-ol; 1-Decanol, 2-hexyl-; 2-Hexyldecanol; 2-Hexyldecyl Alcohol; Guerbitol 16; Isofol 16; Rilanit G 16; Exxal 16; NSC 2399; MFCD00060903; NSC-2399; 151Z7P1317; Guerbet Hexadecanol; Guerbet C16; Jarcol I 16; NJCOL 160BRA; NJCOL 160BR; NSC2399; UNII-151Z7P1317; EINECS 219-370-1; 2-octyl-1-octanol; AI3-19964; EC 219-370-1; HEXYLDECANOL [INCI]; JARCOL I-16; DSSTox_CID_21265; DSSTox_RID_79669; DSSTox_GSID_41265; SCHEMBL15863; 2-Hexyl-1-decanol, 97%; NJCOL 160; CHEMBL3560208; DTXSID1041265; CHEBI:183266; Tox21_300799; AKOS015912415; CS-W012764; NCGC00248173-01; NCGC00254703-01; AS-46984; SY034340; CAS-2425-77-6; FT-0669198; H1461; F11131; A877978; J-509560; Q27251672
|
|
| CAS | 2425-77-6 | |
| PubChem CID | 95337 | |
| ChEMBL ID | CHEMBL3560208 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 242.44 | ALogp: | 7.0 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 13 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 17 | QED Weighted: | 0.419 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.484 | MDCK Permeability: | 0.00001350 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.192 |
| 30% Bioavailability (F30%): | 0.974 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.077 | Plasma Protein Binding (PPB): | 98.10% |
| Volume Distribution (VD): | 2.429 | Fu: | 1.59% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.578 | CYP1A2-substrate: | 0.212 |
| CYP2C19-inhibitor: | 0.415 | CYP2C19-substrate: | 0.078 |
| CYP2C9-inhibitor: | 0.213 | CYP2C9-substrate: | 0.839 |
| CYP2D6-inhibitor: | 0.231 | CYP2D6-substrate: | 0.107 |
| CYP3A4-inhibitor: | 0.313 | CYP3A4-substrate: | 0.07 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.515 | Half-life (T1/2): | 0.177 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.176 | Human Hepatotoxicity (H-HT): | 0.016 |
| Drug-inuced Liver Injury (DILI): | 0.031 | AMES Toxicity: | 0.005 |
| Rat Oral Acute Toxicity: | 0.013 | Maximum Recommended Daily Dose: | 0.024 |
| Skin Sensitization: | 0.945 | Carcinogencity: | 0.034 |
| Eye Corrosion: | 0.974 | Eye Irritation: | 0.959 |
| Respiratory Toxicity: | 0.436 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001235 | 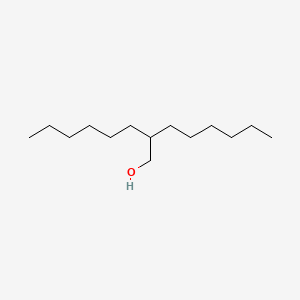 |
0.875 | D07ILQ |  |
0.421 | ||
| ENC000272 | 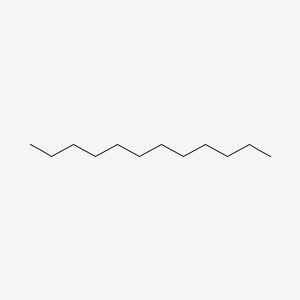 |
0.640 | D05ATI |  |
0.418 | ||
| ENC000421 | 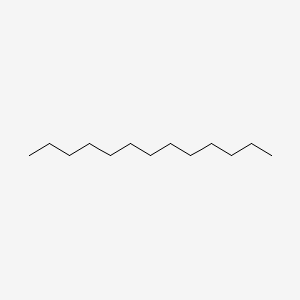 |
0.635 | D0Z5SM |  |
0.417 | ||
| ENC000517 |  |
0.632 | D0O1PH |  |
0.390 | ||
| ENC000422 |  |
0.630 | D00AOJ |  |
0.386 | ||
| ENC000968 |  |
0.627 | D00MLW |  |
0.361 | ||
| ENC000423 |  |
0.625 | D0T9TJ |  |
0.352 | ||
| ENC003063 |  |
0.614 | D0XN8C |  |
0.338 | ||
| ENC000473 |  |
0.612 | D0P1RL |  |
0.337 | ||
| ENC001247 |  |
0.607 | D00FGR |  |
0.337 | ||