NPs Basic Information
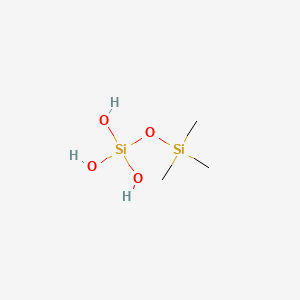
|
Name |
Silicic acid, trimethylsilyl ester
|
| Molecular Formula | C3H12O4Si2 | |
| IUPAC Name* |
trihydroxy(trimethylsilyloxy)silane
|
|
| SMILES |
C[Si](C)(C)O[Si](O)(O)O
|
|
| InChI |
InChI=1S/C3H12O4Si2/c1-8(2,3)7-9(4,5)6/h4-6H,1-3H3
|
|
| InChIKey |
MNTALGTVSQTALQ-UHFFFAOYSA-N
|
|
| Synonyms |
Silicic acid, trimethylsilyl ester; Trimethylsilyl silicate; 56275-01-5; trihydroxy(trimethylsilyloxy)silane; 104133-09-7; OU6EUR7UAY; Trimethylsilyl trihydrogen silicate; Silicic acid, mono(trimethylsilyl) ester; 1,1,1-Disiloxanetriol, 3,3,3-trimethyl-; or MQ resin; 101649-59-6; UNII-25LXE464L2; UNII-5041RX63GN; Trimethylsiloxysilicate (m/q 0.6-0.8); UNII-OU6EUR7UAY; Trimethylsiloxysilanetriol; DSSTox_CID_24958; DSSTox_RID_80614; DSSTox_GSID_44958; SCHEMBL216545; CHEMBL3181894; silicic acid trimethyl-silyl ester; 25LXE464L2; 5041RX63GN; Tox21_301804; NCGC00256297-01; Trimethylsiloxysilicate (m/q 0.8-1.0); CAS-56275-01-5; 161035-75-2; 754999-67-2
|
|
| CAS | 56275-01-5 | |
| PubChem CID | 91783 | |
| ChEMBL ID | CHEMBL3181894 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 168.3 | ALogp: | -0.7 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 69.9 | Aromatic Rings: | 0 |
| Heavy Atoms: | 9 | QED Weighted: | 0.496 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.654 | MDCK Permeability: | 0.00673502 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.019 |
| Human Intestinal Absorption (HIA): | 0.18 | 20% Bioavailability (F20%): | 0.04 |
| 30% Bioavailability (F30%): | 0.103 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.018 | Plasma Protein Binding (PPB): | 99.18% |
| Volume Distribution (VD): | 1.043 | Fu: | 2.99% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.166 | CYP1A2-substrate: | 0.745 |
| CYP2C19-inhibitor: | 0.042 | CYP2C19-substrate: | 0.117 |
| CYP2C9-inhibitor: | 0.011 | CYP2C9-substrate: | 0.928 |
| CYP2D6-inhibitor: | 0.018 | CYP2D6-substrate: | 0.672 |
| CYP3A4-inhibitor: | 0.007 | CYP3A4-substrate: | 0.014 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.054 | Half-life (T1/2): | 0.689 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.033 | Human Hepatotoxicity (H-HT): | 0.054 |
| Drug-inuced Liver Injury (DILI): | 0.012 | AMES Toxicity: | 0.109 |
| Rat Oral Acute Toxicity: | 0.002 | Maximum Recommended Daily Dose: | 0.061 |
| Skin Sensitization: | 0.698 | Carcinogencity: | 0.465 |
| Eye Corrosion: | 0.996 | Eye Irritation: | 0.982 |
| Respiratory Toxicity: | 0.973 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000469 | 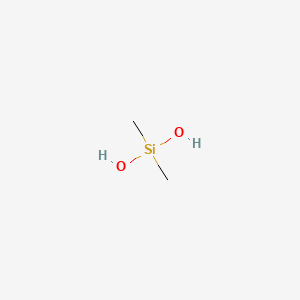 |
0.409 | D06QDR | 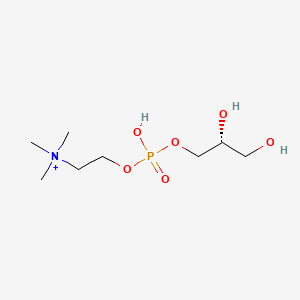 |
0.127 | ||
| ENC001269 | 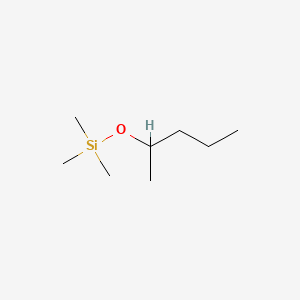 |
0.243 | D0C1QZ | 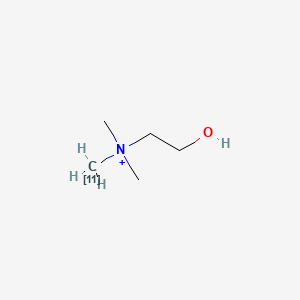 |
0.118 | ||
| ENC001178 | 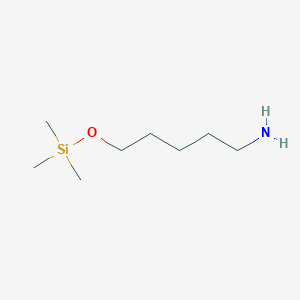 |
0.220 | D0ZK8H | 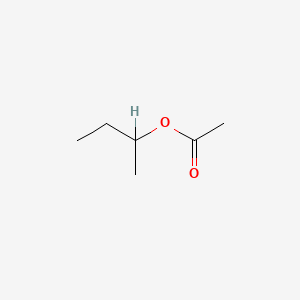 |
0.108 | ||
| ENC001177 | 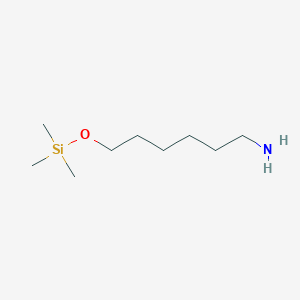 |
0.205 | D02KJX | 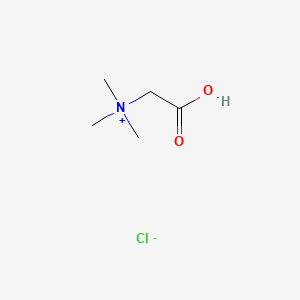 |
0.108 | ||
| ENC000530 | 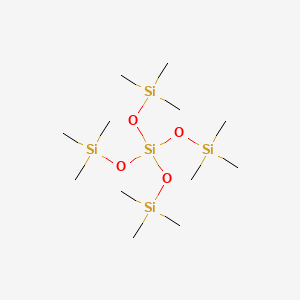 |
0.200 | D0M8RC | 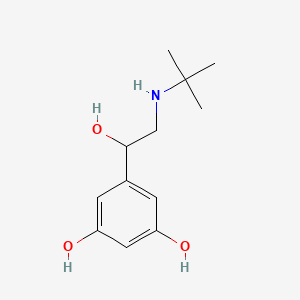 |
0.105 | ||
| ENC001151 | 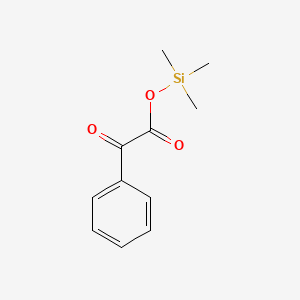 |
0.173 | D07QKN | 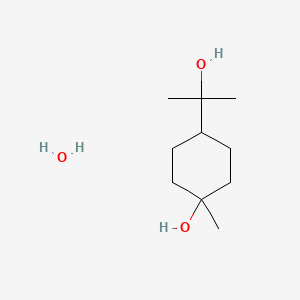 |
0.104 | ||
| ENC001404 | 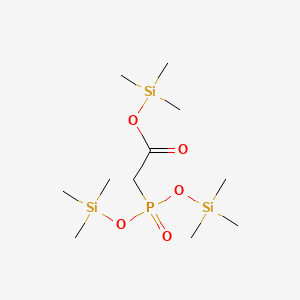 |
0.167 | D0FM2P | 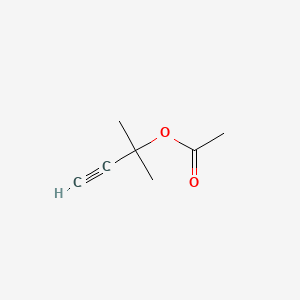 |
0.103 | ||
| ENC001314 | 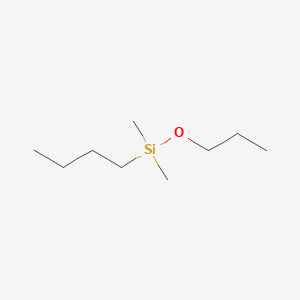 |
0.163 | D0SS4P | 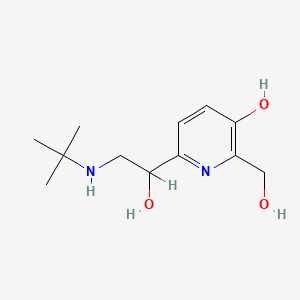 |
0.100 | ||
| ENC000814 | 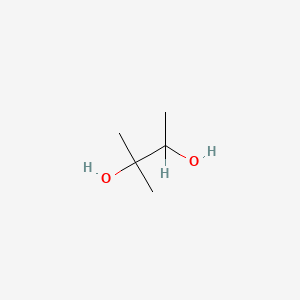 |
0.161 | D02ZJI | 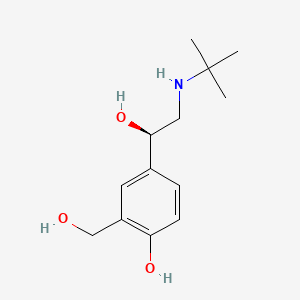 |
0.100 | ||
| ENC000733 | 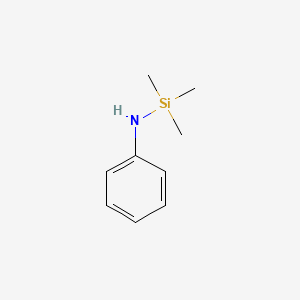 |
0.159 | D0K5CB | 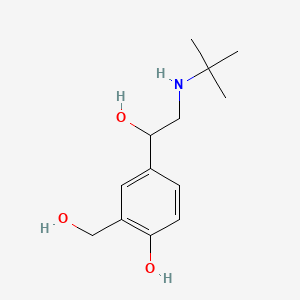 |
0.100 | ||