NPs Basic Information
|
Name |
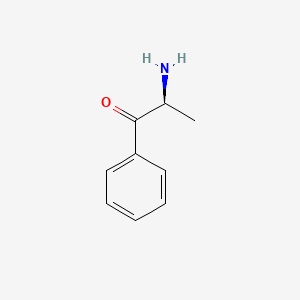
Cathinone
|
| Molecular Formula | C9H11NO | |
| IUPAC Name* |
(2S)-2-amino-1-phenylpropan-1-one
|
|
| SMILES |
C[C@@H](C(=O)C1=CC=CC=C1)N
|
|
| InChI |
InChI=1S/C9H11NO/c1-7(10)9(11)8-5-3-2-4-6-8/h2-7H,10H2,1H3/t7-/m0/s1
|
|
| InChIKey |
PUAQLLVFLMYYJJ-ZETCQYMHSA-N
|
|
| Synonyms |
Cathinone; Norephedrone; d-Cathinone; 71031-15-7; l-Cathinone; (2S)-2-amino-1-phenylpropan-1-one; Cathinone [INN]; Cathinonum; Catinona; (S)-(-)-Cathinone; (S)-2-Aminopropiophenone; (-)-Cathinone; S-(-)-cathinone; alpha-Aminopropiophenone; 1-Propanone, 2-amino-1-phenyl-, (S)-; CHEBI:4110; (-)-.alpha.-Aminopropiophenone; C08301; 540EI4406J; J18.754B; (4R,5S)-4-methyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine; Cathinone (incb:green list); CATHINONE [INCB:GREEN LIST]; Cathinonum [INN-Latin]; Catinona [INN-Spanish]; 1-Propanone, 2-amino-1-phenyl-, (2S)-; NCGC00168262-01; NCGC00168262-02; BRN 5247015; (-)-alpha-Aminopropiophenone; CCRIS 9310; DEA No. 1235; UNII-540EI4406J; S(-)-Cathinone; (2S)-2-Amino-1-phenyl-1-propanone; CATHINONE [MI]; CATHINONE [MART.]; CATHINONE [WHO-DD]; SCHEMBL34513; CATHINONE [USP IMPURITY]; CHEMBL2104047; DTXSID0050427; 2-Amino-1-phenyl-1-propanone #; PDSP1_001350; PDSP2_001334; ZINC53165481; DB01560; Q414242; AMFETAMINE SULFATE IMPURITY C [EP IMPURITY]
|
|
| CAS | 71031-15-7 | |
| PubChem CID | 62258 | |
| ChEMBL ID | CHEMBL2104047 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 149.19 | ALogp: | 1.1 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 43.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.65 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.908 | MDCK Permeability: | 0.00006730 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.059 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.984 | Plasma Protein Binding (PPB): | 43.41% |
| Volume Distribution (VD): | 2.914 | Fu: | 69.35% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.736 | CYP1A2-substrate: | 0.103 |
| CYP2C19-inhibitor: | 0.061 | CYP2C19-substrate: | 0.35 |
| CYP2C9-inhibitor: | 0.014 | CYP2C9-substrate: | 0.065 |
| CYP2D6-inhibitor: | 0.424 | CYP2D6-substrate: | 0.557 |
| CYP3A4-inhibitor: | 0.079 | CYP3A4-substrate: | 0.228 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.665 | Half-life (T1/2): | 0.753 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.096 | Human Hepatotoxicity (H-HT): | 0.139 |
| Drug-inuced Liver Injury (DILI): | 0.093 | AMES Toxicity: | 0.047 |
| Rat Oral Acute Toxicity: | 0.832 | Maximum Recommended Daily Dose: | 0.039 |
| Skin Sensitization: | 0.154 | Carcinogencity: | 0.075 |
| Eye Corrosion: | 0.012 | Eye Irritation: | 0.058 |
| Respiratory Toxicity: | 0.494 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000076 | 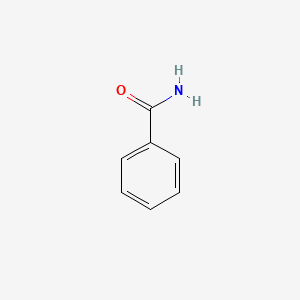 |
0.618 | D0X9RY |  |
0.571 | ||
| ENC000192 | 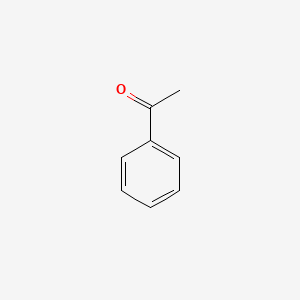 |
0.618 | D0B7OD | 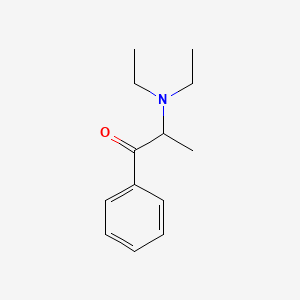 |
0.543 | ||
| ENC000013 | 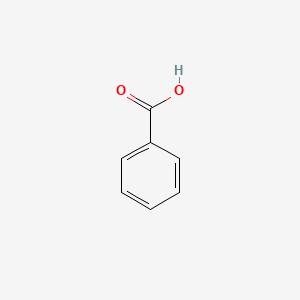 |
0.571 | D0T3LF | 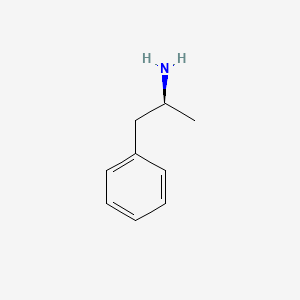 |
0.487 | ||
| ENC000174 | 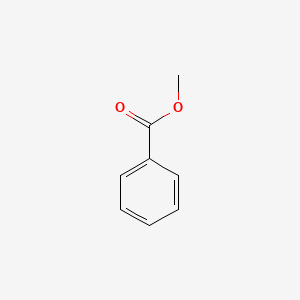 |
0.568 | D05BMG |  |
0.487 | ||
| ENC000637 | 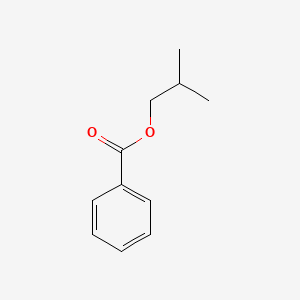 |
0.535 | D0R1CR |  |
0.465 | ||
| ENC000175 | 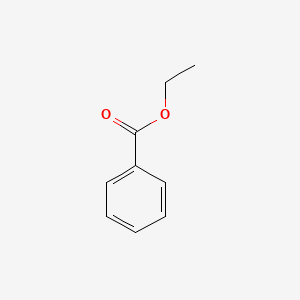 |
0.525 | D0S7VO | 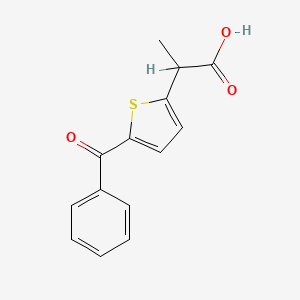 |
0.404 | ||
| ENC000176 | 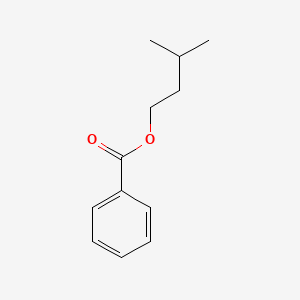 |
0.500 | D0P6UB | 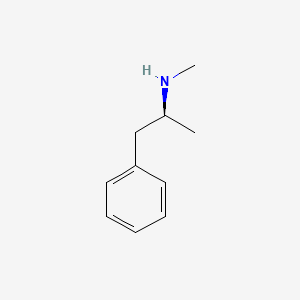 |
0.386 | ||
| ENC000130 | 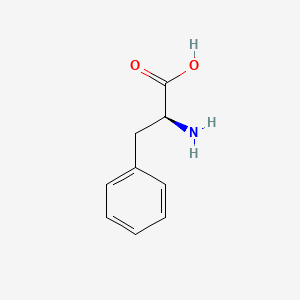 |
0.465 | D01ZJK |  |
0.386 | ||
| ENC001914 | 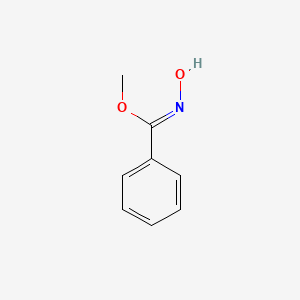 |
0.452 | D0W9WF | 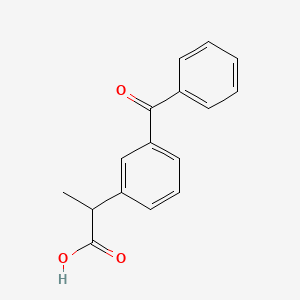 |
0.383 | ||
| ENC000219 | 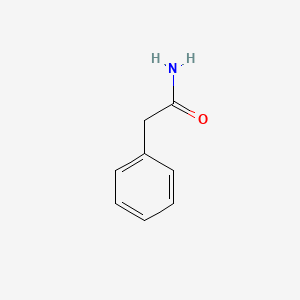 |
0.450 | D07ONP | 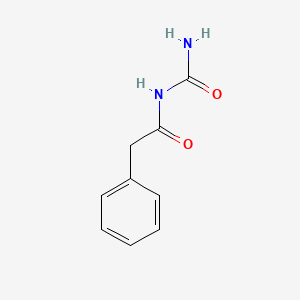 |
0.375 | ||