NPs Basic Information
|
Name |
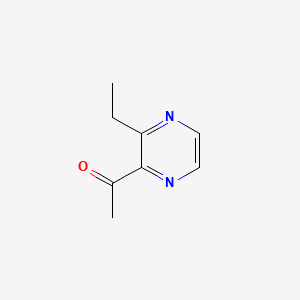
2-Acetyl-3-ethylpyrazine
|
| Molecular Formula | C8H10N2O | |
| IUPAC Name* |
1-(3-ethylpyrazin-2-yl)ethanone
|
|
| SMILES |
CCC1=NC=CN=C1C(=O)C
|
|
| InChI |
InChI=1S/C8H10N2O/c1-3-7-8(6(2)11)10-5-4-9-7/h4-5H,3H2,1-2H3
|
|
| InChIKey |
PPJSYGVFDJEMRP-UHFFFAOYSA-N
|
|
| Synonyms |
2-Acetyl-3-ethylpyrazine; 32974-92-8; 1-(3-ethylpyrazin-2-yl)ethanone; 1-(3-Ethylpyrazinyl)ethan-1-one; Ethanone, 1-(3-ethylpyrazinyl)-; 2-Acetyl-3-ethyl-1,4-diazine; 2-acetyl-3-ethyl pyrazine; 1-(3-ethylpyrazin-2-yl)ethan-1-one; 1-(3-Ethylpyrazinyl)ethanone; FEMA No. 3250; Ethanone, 1-(ethylpyrazinyl)-; 3-Ethyl-2-acetylpyrazine; Ethanone, 1-(3-ethyl-2-pyrazinyl)-; 2-Ethyl-3-acetyl pyrazine; AQ03N14XTL; Pyrazine, 2-acetyl-3-ethyl; 2-ACETYL ETHYLPYRAZINE; 1-(3-Ethyl-2-pyrazinyl)ethanone; MFCD00038028; UNII-AQ03N14XTL; EINECS 251-316-2; acetyl-3-ethylpyrazine, 2-; SCHEMBL1532459; 1-(3-Ethylpyrazinyl)-Ethanone; DTXSID3067736; FEMA 3250; CHEBI:173401; ZINC1850550; 1-(3-Ethyl-2-pyrazinyl)-Ethanone; 1-(3-Ethylpyrazinyl)ethanone, 9CI; AC8556; 1-(3-Ethyl-pyrazin-2-yl)-ethanone; 2-Acetyl-3-ethylpyrazine, 98%, FG; AKOS005256376; FS-1047; HY-W039157; 2-ACETYL-3-ETHYLPYRAZINE [FHFI]; SY027477; DB-020324; CS-0096817; FT-0610950; 974A928; A821538; Q-100406; Q27274053; 1-(3-ethylpyrazin-2-yl)ethanone;2-Acetyl-3-ethylpyrazine
|
|
| CAS | 32974-92-8 | |
| PubChem CID | 61918 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 150.18 | ALogp: | 0.6 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 42.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.603 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.387 | MDCK Permeability: | 0.00003590 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.742 | Plasma Protein Binding (PPB): | 29.44% |
| Volume Distribution (VD): | 1.386 | Fu: | 71.89% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.688 | CYP1A2-substrate: | 0.707 |
| CYP2C19-inhibitor: | 0.079 | CYP2C19-substrate: | 0.698 |
| CYP2C9-inhibitor: | 0.014 | CYP2C9-substrate: | 0.676 |
| CYP2D6-inhibitor: | 0.005 | CYP2D6-substrate: | 0.466 |
| CYP3A4-inhibitor: | 0.01 | CYP3A4-substrate: | 0.238 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.817 | Half-life (T1/2): | 0.446 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.525 |
| Drug-inuced Liver Injury (DILI): | 0.907 | AMES Toxicity: | 0.043 |
| Rat Oral Acute Toxicity: | 0.665 | Maximum Recommended Daily Dose: | 0.438 |
| Skin Sensitization: | 0.862 | Carcinogencity: | 0.178 |
| Eye Corrosion: | 0.162 | Eye Irritation: | 0.987 |
| Respiratory Toxicity: | 0.797 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000577 | 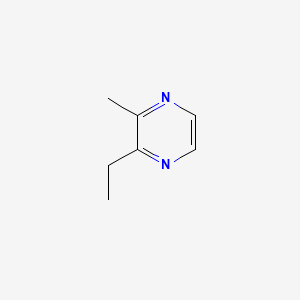 |
0.571 | D0XF8W |  |
0.279 | ||
| ENC000612 | 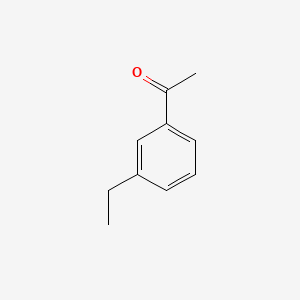 |
0.304 | D0P0HB | 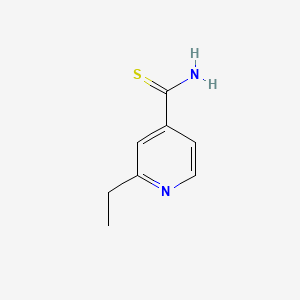 |
0.250 | ||
| ENC001141 | 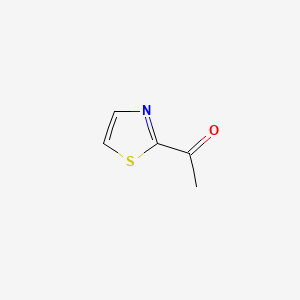 |
0.300 | D0L7UQ | 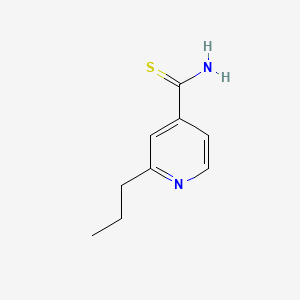 |
0.212 | ||
| ENC005053 | 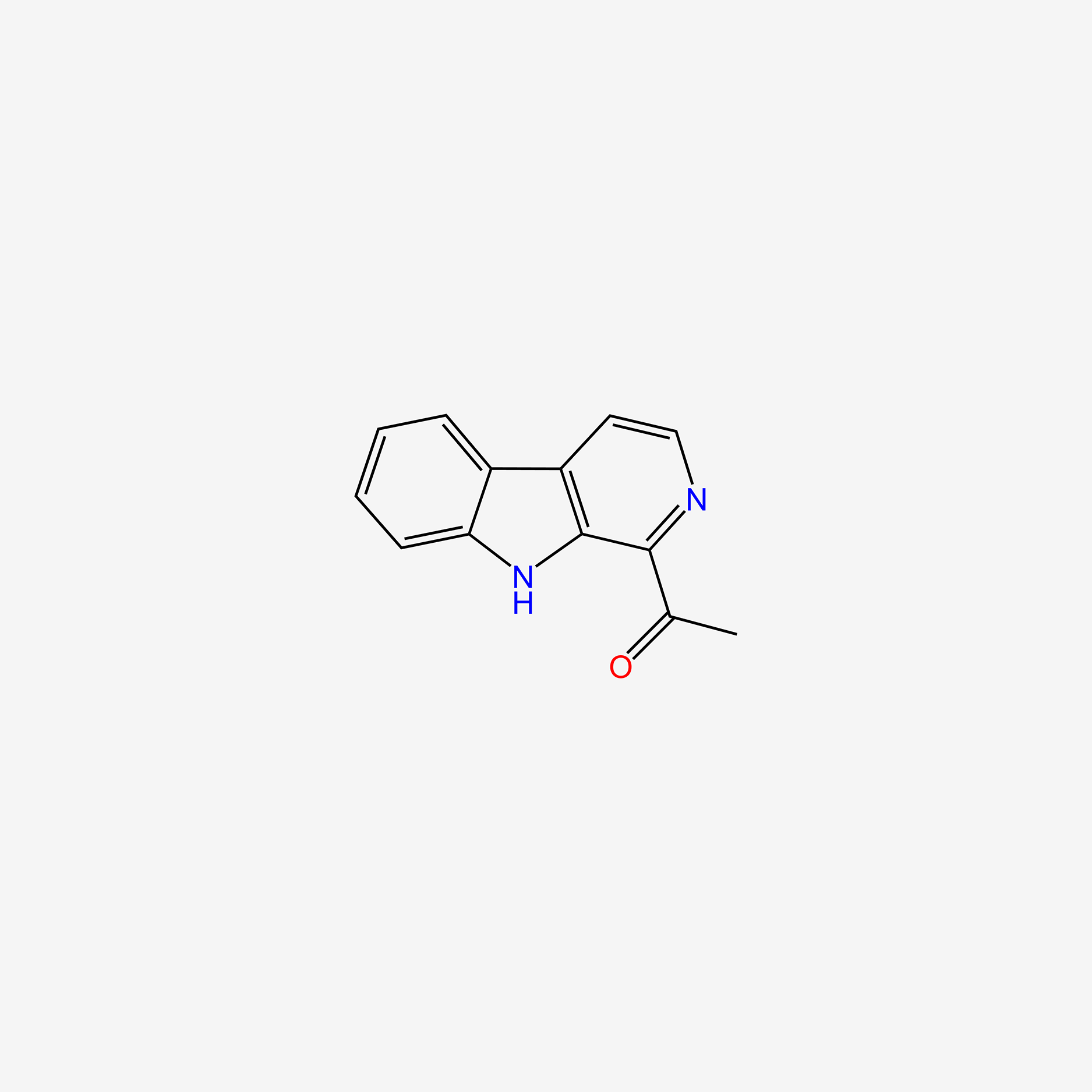 |
0.267 | D0S1NZ | 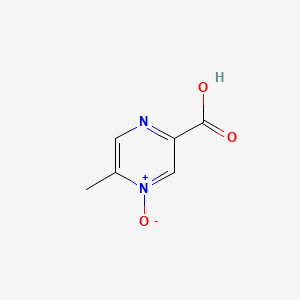 |
0.204 | ||
| ENC000599 | 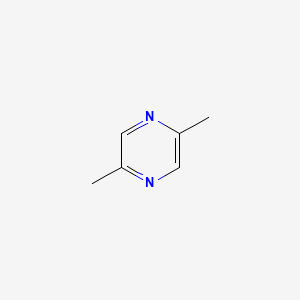 |
0.238 | D09DWL | 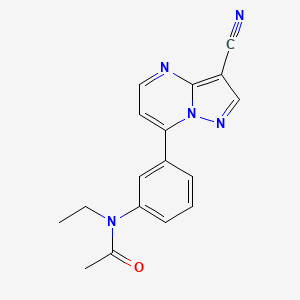 |
0.203 | ||
| ENC000640 | 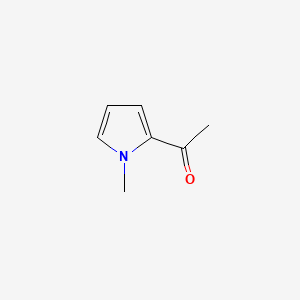 |
0.227 | D0Q9JT | 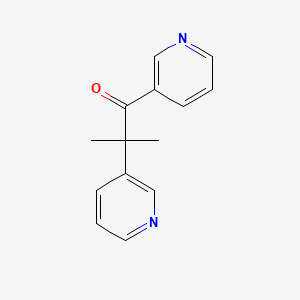 |
0.200 | ||
| ENC000201 | 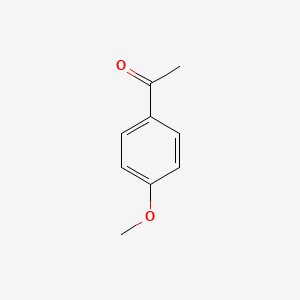 |
0.224 | D0C8EU | 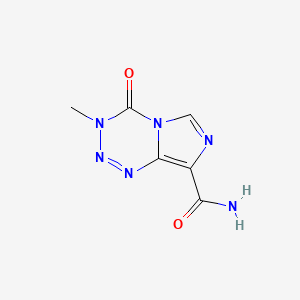 |
0.193 | ||
| ENC000391 | 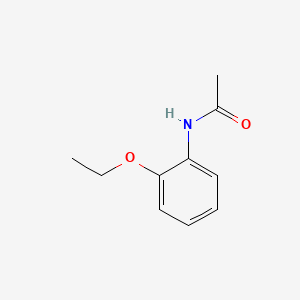 |
0.222 | D02CKX | 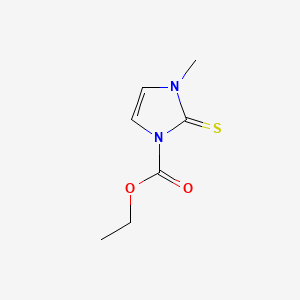 |
0.192 | ||
| ENC000106 | 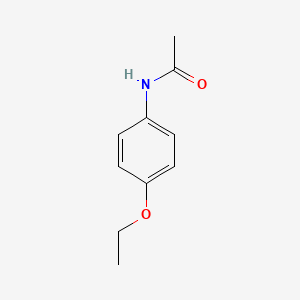 |
0.222 | D0ZK8H | 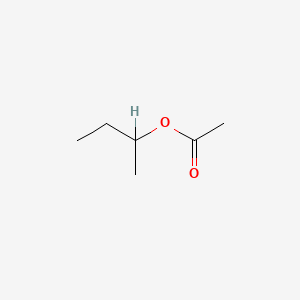 |
0.190 | ||
| ENC000096 | 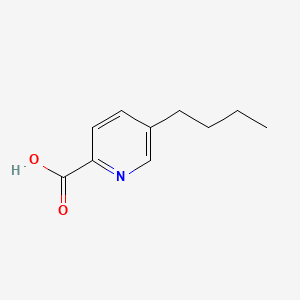 |
0.222 | D0VT8P | 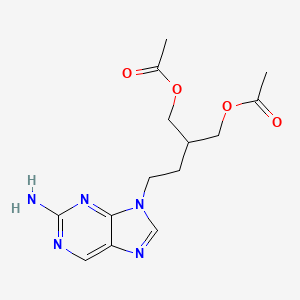 |
0.190 | ||