NPs Basic Information
|
Name |
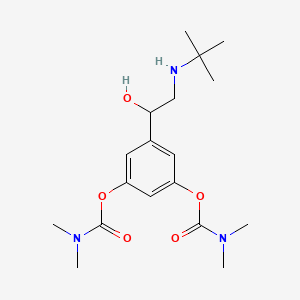
Bambuterol
|
| Molecular Formula | C18H29N3O5 | |
| IUPAC Name* |
[3-[2-(tert-butylamino)-1-hydroxyethyl]-5-(dimethylcarbamoyloxy)phenyl] N,N-dimethylcarbamate
|
|
| SMILES |
CC(C)(C)NCC(C1=CC(=CC(=C1)OC(=O)N(C)C)OC(=O)N(C)C)O
|
|
| InChI |
InChI=1S/C18H29N3O5/c1-18(2,3)19-11-15(22)12-8-13(25-16(23)20(4)5)10-14(9-12)26-17(24)21(6)7/h8-10,15,19,22H,11H2,1-7H3
|
|
| InChIKey |
ANZXOIAKUNOVQU-UHFFFAOYSA-N
|
|
| Synonyms |
Bambuterol; 81732-65-2; Bambec; Bambuterolum; Bambuterol (INN); CHEBI:553827; [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-(dimethylcarbamoyloxy)phenyl] N,N-dimethylcarbamate; Y1850G1OVC; (+/-)-Bambuterol;KWD-2183; (+-)-5-(2-(tert-Butylamino)-1-hydroxyethyl)-m-phenylene bis(dimethylcarbamate); Bambuterolum [Latin]; Oxeol; BAMBUTEROL [INN]; KWD-2183; 5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diyl bis(dimethylcarbamate); (+/-)-5-(2-(tert-butylamino)-1-hydroxyethyl)-m-phenylene bis(dimethylcarbamate); terbutaline bisdimethylcarbamate; Bambuterol [INN:BAN]; UNII-Y1850G1OVC; terbutaline bis(dimethylcarbamate); BAMBUTEROL [MI]; Prestwick0_000361; Prestwick1_000361; Prestwick2_000361; Prestwick3_000361; SCHEMBL4431; BAMBUTEROL [WHO-DD]; BSPBio_000481; MLS002153785; SPBio_002402; BPBio1_000531; CHEMBL521589; GTPL6601; DTXSID5048550; GLXC-25236; HMS2089J18; HMS2230O15; HMS3373N13; BCP21793; BDBM50235800; STK643808; AKOS005574764; CS-3157; DB01408; ( inverted exclamation markA)-Bambutero; ( inverted exclamation markA)-Bambuterol; NCGC00179546-01; NCGC00179546-02; AC-35438; HY-17501; SMR001233168; SBI-0207028.P001; FT-0602901; D07377; 732B652; A840189; L004435; Q3633651; SR-05000001470-1; BRD-A17462676-003-03-3; BRD-A17462676-003-06-6; (RS)-5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diyl bis(dimethylcarbamate); 5-(2-(tert-butylamino)-1-hydroxyethyl)-1,3-phenylene bis(dimethylcarbamate); [3-[2-(tert-butylamino)-1-oxidanyl-ethyl]-5-(dimethylcarbamoyloxy)phenyl] N,N-dimethylcarbamate; N,N-dimethylcarbamic acid [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-[dimethylamino(oxo)methoxy]phenyl] ester
|
|
| CAS | 81732-65-2 | |
| PubChem CID | 54766 | |
| ChEMBL ID | CHEMBL521589 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 367.4 | ALogp: | 1.2 |
| HBD: | 2 | HBA: | 6 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 91.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 26 | QED Weighted: | 0.831 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.793 | MDCK Permeability: | 0.00001920 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.865 |
| Human Intestinal Absorption (HIA): | 1 | 20% Bioavailability (F20%): | 0.997 |
| 30% Bioavailability (F30%): | 0.992 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.166 | Plasma Protein Binding (PPB): | 37.89% |
| Volume Distribution (VD): | 0.778 | Fu: | 74.54% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.023 | CYP1A2-substrate: | 0.086 |
| CYP2C19-inhibitor: | 0.024 | CYP2C19-substrate: | 0.969 |
| CYP2C9-inhibitor: | 0.002 | CYP2C9-substrate: | 0.134 |
| CYP2D6-inhibitor: | 0.724 | CYP2D6-substrate: | 0.753 |
| CYP3A4-inhibitor: | 0.033 | CYP3A4-substrate: | 0.915 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.92 | Half-life (T1/2): | 0.878 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.122 | Human Hepatotoxicity (H-HT): | 0.006 |
| Drug-inuced Liver Injury (DILI): | 0.039 | AMES Toxicity: | 0.068 |
| Rat Oral Acute Toxicity: | 0.965 | Maximum Recommended Daily Dose: | 0.938 |
| Skin Sensitization: | 0.315 | Carcinogencity: | 0.037 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.007 |
| Respiratory Toxicity: | 0.937 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004204 | 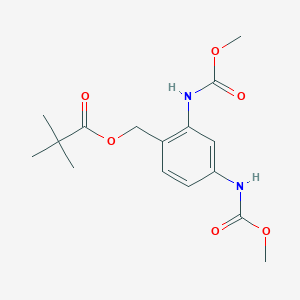 |
0.245 | D07XYV | 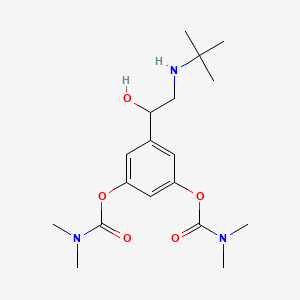 |
1.000 | ||
| ENC000658 | 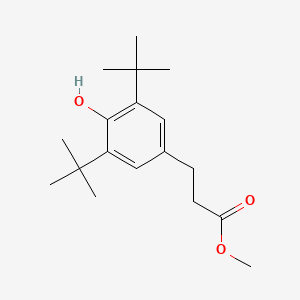 |
0.245 | D0M8RC | 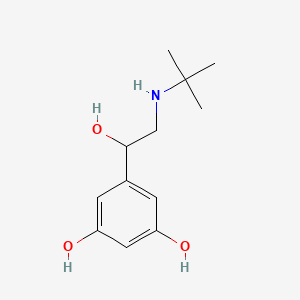 |
0.392 | ||
| ENC001392 |  |
0.241 | D01JFT | 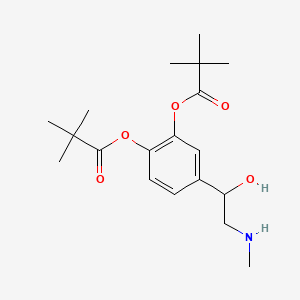 |
0.385 | ||
| ENC001382 | 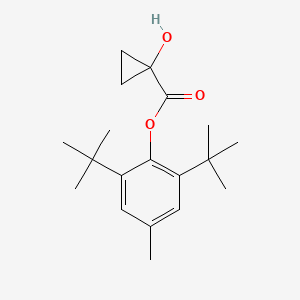 |
0.240 | D0X5NX | 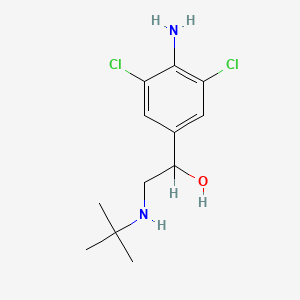 |
0.349 | ||
| ENC000695 |  |
0.233 | D0AY7K |  |
0.330 | ||
| ENC003608 |  |
0.228 | D0K5CB |  |
0.329 | ||
| ENC000071 | 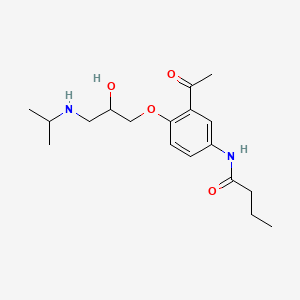 |
0.220 | D02ZJI | 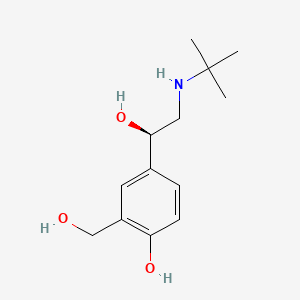 |
0.329 | ||
| ENC002963 | 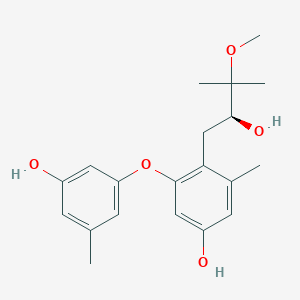 |
0.216 | D06RUL | 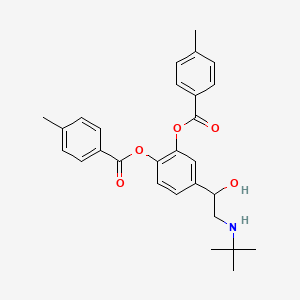 |
0.328 | ||
| ENC006118 | 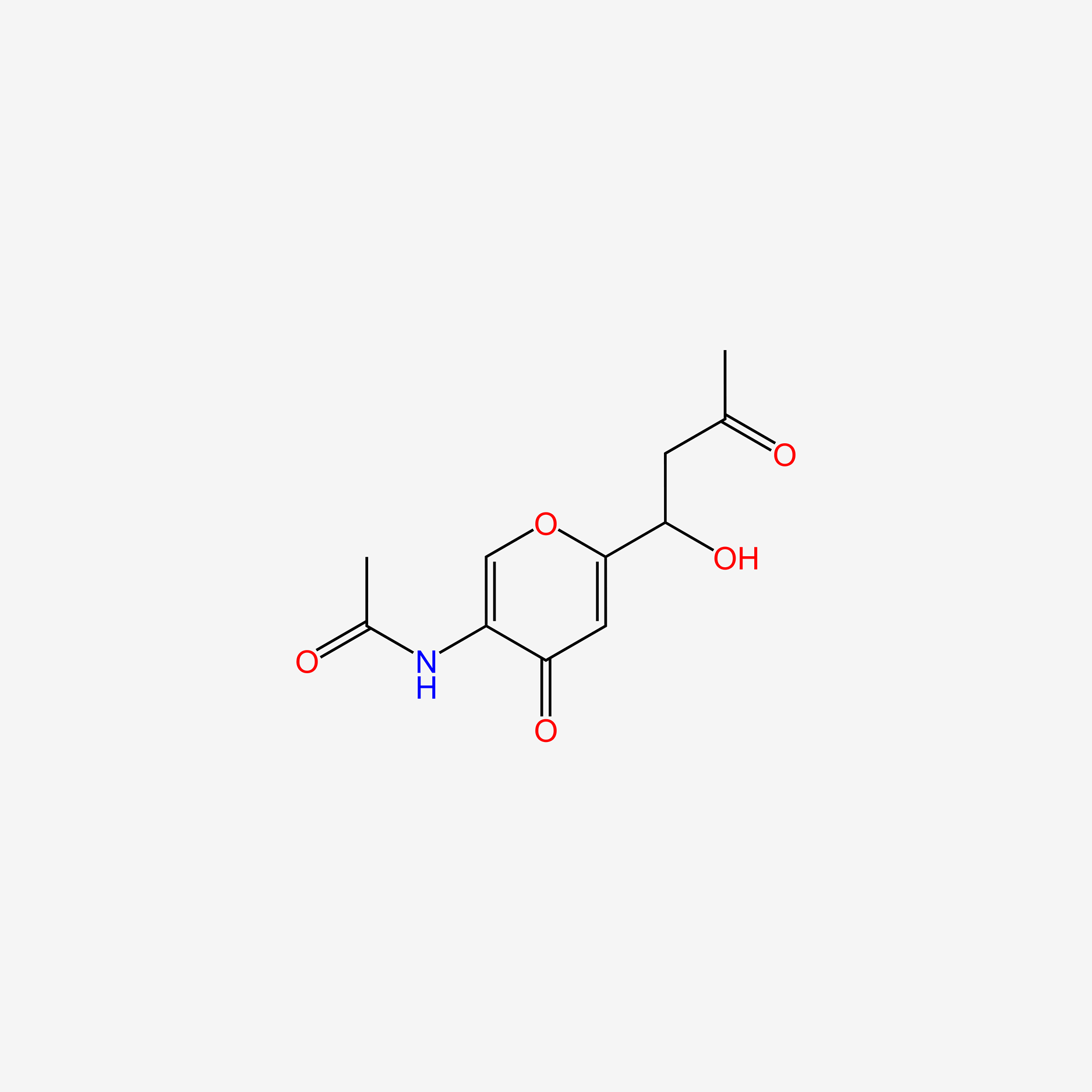 |
0.215 | D0WY5Q | 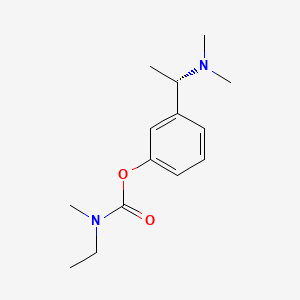 |
0.318 | ||
| ENC003377 | 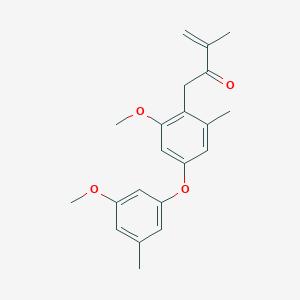 |
0.214 | D08USJ | 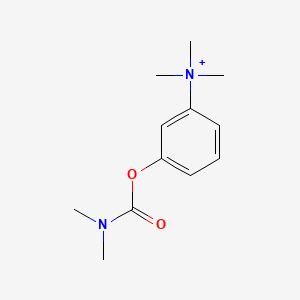 |
0.294 | ||