NPs Basic Information
|
Name |
Timolol
|
| Molecular Formula | C13H24N4O3S | |
| IUPAC Name* |
(2S)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol
|
|
| SMILES |
CC(C)(C)NC[C@@H](COC1=NSN=C1N2CCOCC2)O
|
|
| InChI |
InChI=1S/C13H24N4O3S/c1-13(2,3)14-8-10(18)9-20-12-11(15-21-16-12)17-4-6-19-7-5-17/h10,14,18H,4-9H2,1-3H3/t10-/m0/s1
|
|
| InChIKey |
BLJRIMJGRPQVNF-JTQLQIEISA-N
|
|
| Synonyms |
timolol; 26839-75-8; (S)-timolol; Istalol; Timolol anhydrous; (2S)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol; Timoptic-XE; (S)-timolol (anhydrous); Timololum [INN-Latin]; (-)-3-Morpholino-4-(3-tert-butylamino-2-hydroxypropoxy)-1,2,5-thiadiazole; S-(-)-3-(3-tert-Butylamino-2-hydroxypropoxy)-4-morpholino-1,2,5-thiadiazole; Timolol (INN); (S)-1-(1,1-(Dimethylethyl)amino)-3-((4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl)oxy)-2-propanol; (S)-1-(tert-Butylamino)-3-((4-morpholino-1,2,5-thiadiazol-3-yl)oxy)propan-2-ol; Betimol (TN); Timopic; CHEMBL499; 5JKY92S7BR; CHEBI:9599; Timolol GFS; (2S)-1-((1,1-dimethylethyl)amino)-3-((4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl)oxy)-2-propanol; NCGC00016798-06; Timololum; TIMOLOL [INN]; DSSTox_CID_3674; DSSTox_RID_77140; DSSTox_GSID_23674; 2-Propanol, 1-((1,1-dimethylethyl)amino)-3-((4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl)oxy)-, (2S)-; 2-Propanol, 1-[(1,1-dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-, (2S)-; Timolol (TN); CAS-26839-75-8; HSDB 6533; Timolol [USAN:INN]; EINECS 248-032-6; CAS-26921-17-5; UNII-5JKY92S7BR; Tocris-0649; TIMOLOL [HSDB]; TIMOLOL [MI]; Prestwick0_000948; Prestwick1_000948; Prestwick2_000948; Prestwick3_000948; Lopac-T-6394; TIMOLOL [WHO-DD]; SCHEMBL4912; Lopac0_001189; Oprea1_640981; BSPBio_000916; GTPL565; 2-Propanol, 1-((1,1-dimethylethyl)amino)-3-((4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl)oxy)-, (S)-; BIDD:GT0073; SPBio_003075; BPBio1_001008; ZINC2176; DTXSID4023674; HMS2089I11; HMS3259C20; Tox21_110614; BDBM50292219; AKOS015969764; Tox21_110614_1; DB00373; NC00592; (2S)-1-(tert-butylamino)-3-{[4-(morpholin-4-yl)-1,2,5-thiadiazol-3-yl]oxy}propan-2-ol; (2S)-3-[(tert-butyl)amino]-1-(4-morpholin-4-yl(1,2,5-thiadiazol-3-yl)oxy)propa n-2-ol; NCGC00016038-01; NCGC00016038-02; NCGC00016798-01; NCGC00016798-02; NCGC00016798-03; NCGC00016798-05; NCGC00016798-07; NCGC00016798-08; NCGC00016798-09; NCGC00016798-11; NCGC00016798-20; NCGC00022033-02; NCGC00022033-04; NCGC00022033-05; (2S)-1-[(1,1-dimethylethyl)amino]-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol; HY-17494; AB00513729; CS-0009238; C07141; D08600; AB00513729-17; 839T758; EN300-17982937; Q413994; W-107148; BRD-K08806317-050-03-6; BRD-K08806317-103-02-5; (S)-1-(TERT-BUTYLAMINO)-3-((4-MORPHOLINO-1,2,5-THIADIAZOL-3-YL)OXY)-2-PROPANOL; (S)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol; (S)-1-tert-Butylamino-3-(4-morpholin-4-yl-[1,2,5]thiadiazol-3-yloxy)-propan-2-ol; 1-(tert-butylamino)-3-[4-(1,4-oxazinan-4-yl)-1,2,5-thiadiazol-3-yloxy]-(2S)-propan-2-ol
|
|
| CAS | 26839-75-8 | |
| PubChem CID | 33624 | |
| ChEMBL ID | CHEMBL499 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 316.42 | ALogp: | 1.8 |
| HBD: | 2 | HBA: | 8 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 108.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 21 | QED Weighted: | 0.807 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.712 | MDCK Permeability: | 0.00001910 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.03 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.596 | Plasma Protein Binding (PPB): | 13.07% |
| Volume Distribution (VD): | 0.859 | Fu: | 87.54% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.039 | CYP1A2-substrate: | 0.096 |
| CYP2C19-inhibitor: | 0.057 | CYP2C19-substrate: | 0.911 |
| CYP2C9-inhibitor: | 0.005 | CYP2C9-substrate: | 0.113 |
| CYP2D6-inhibitor: | 0.888 | CYP2D6-substrate: | 0.878 |
| CYP3A4-inhibitor: | 0.145 | CYP3A4-substrate: | 0.78 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.264 | Half-life (T1/2): | 0.913 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.021 | Human Hepatotoxicity (H-HT): | 0.814 |
| Drug-inuced Liver Injury (DILI): | 0.024 | AMES Toxicity: | 0.139 |
| Rat Oral Acute Toxicity: | 0.005 | Maximum Recommended Daily Dose: | 0.948 |
| Skin Sensitization: | 0.954 | Carcinogencity: | 0.96 |
| Eye Corrosion: | 0.005 | Eye Irritation: | 0.012 |
| Respiratory Toxicity: | 0.79 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
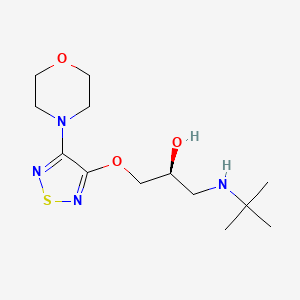
| ENC000244 |  |
0.234 | D05UVD | 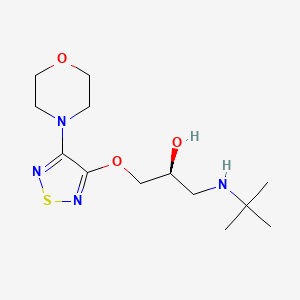 |
1.000 | ||
| ENC001185 |  |
0.200 | D03SFU | 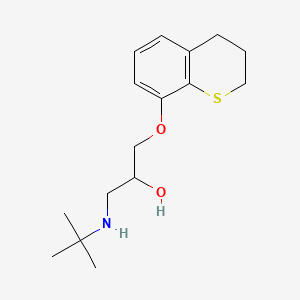 |
0.369 | ||
| ENC000634 | 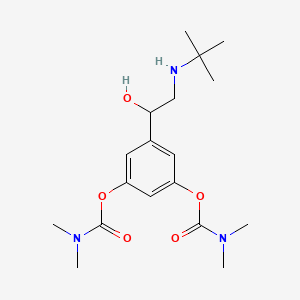 |
0.187 | D0W8SB | 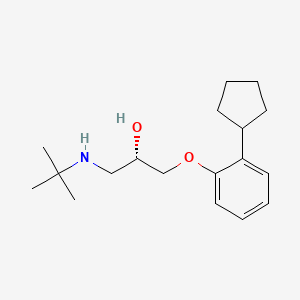 |
0.356 | ||
| ENC004066 | 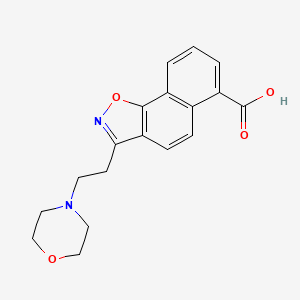 |
0.183 | D00IUG | 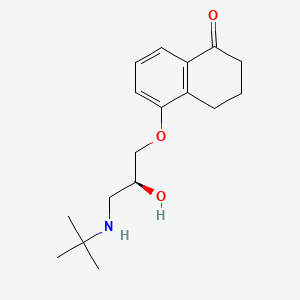 |
0.345 | ||
| ENC000074 | 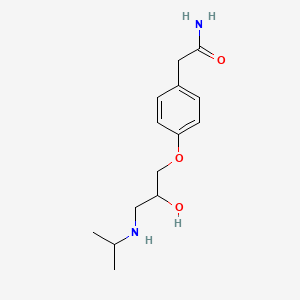 |
0.181 | D03GCJ | 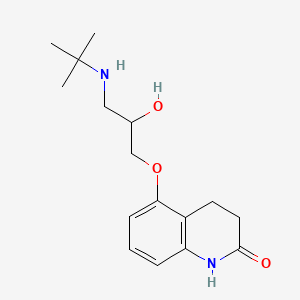 |
0.330 | ||
| ENC000071 | 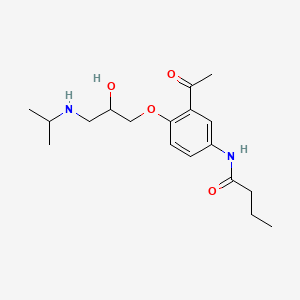 |
0.170 | D05SHK | 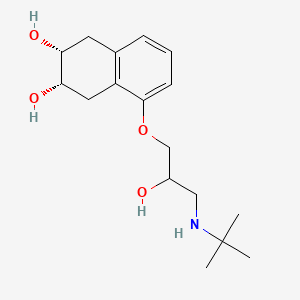 |
0.322 | ||
| ENC005607 | 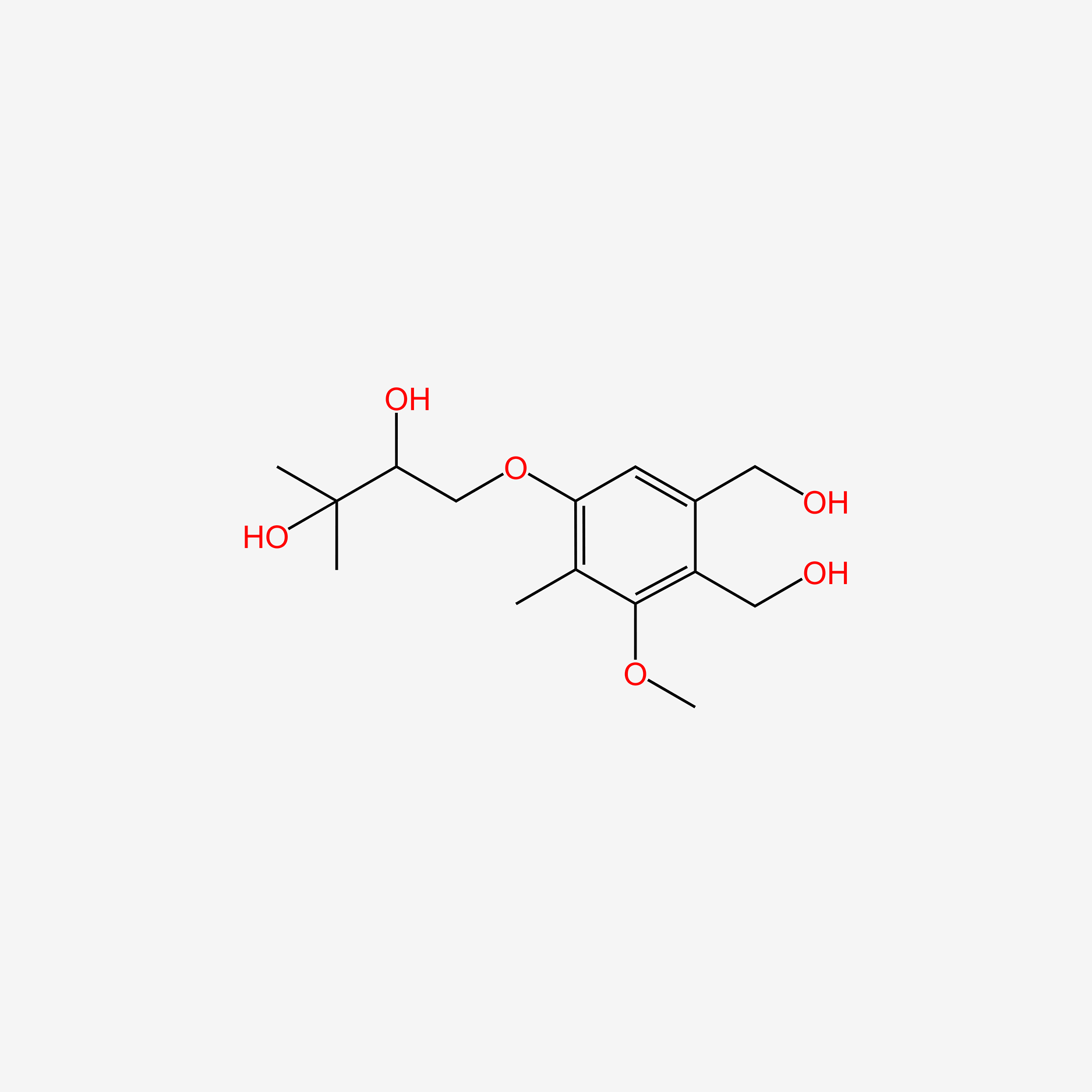 |
0.163 | D0AY7K | 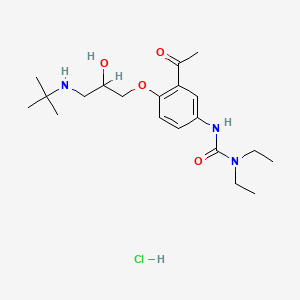 |
0.257 | ||
| ENC005263 | 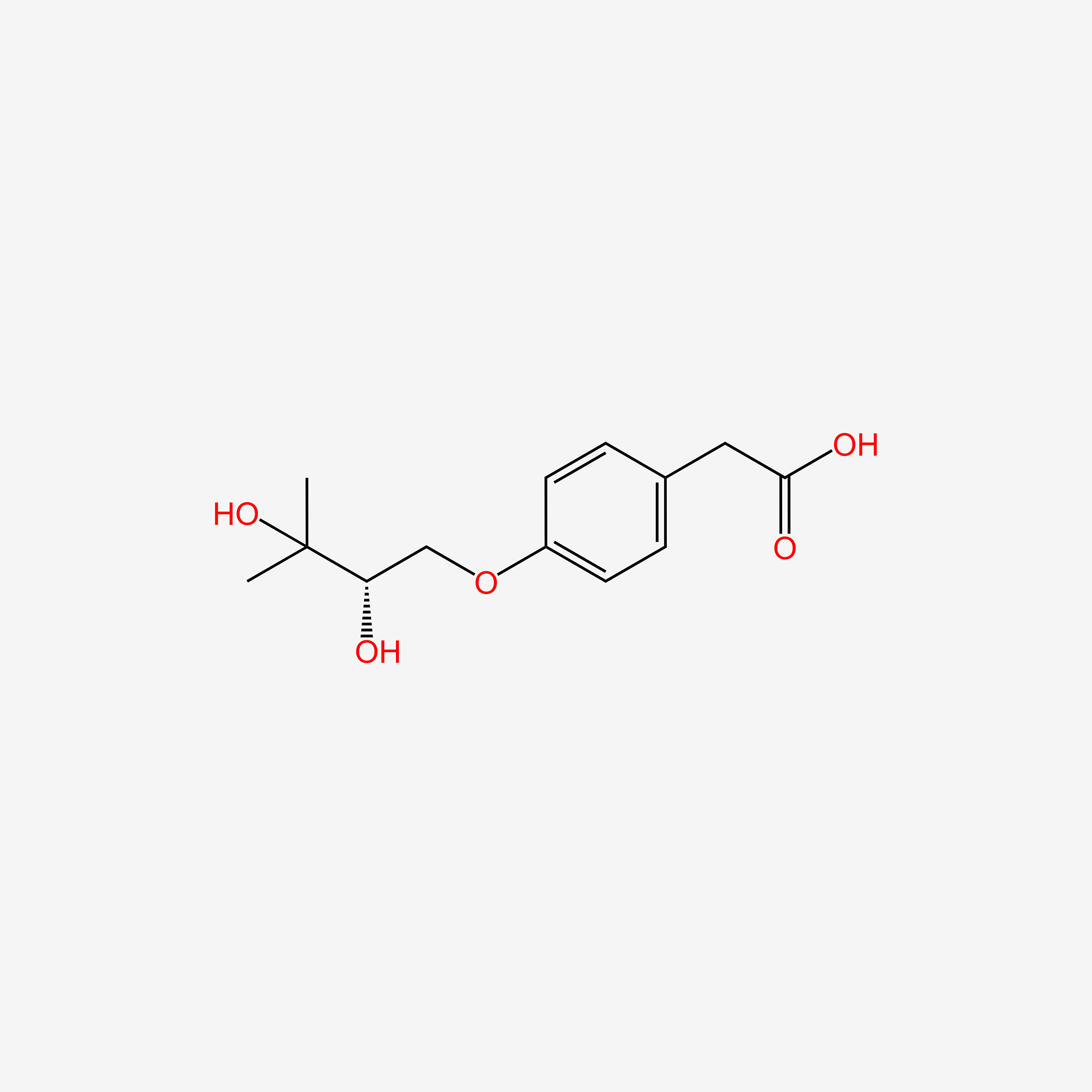 |
0.163 | D0SS4P | 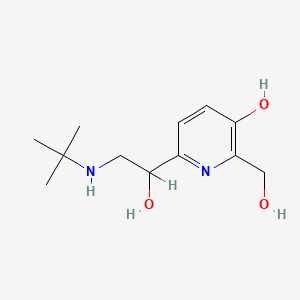 |
0.253 | ||
| ENC005826 | 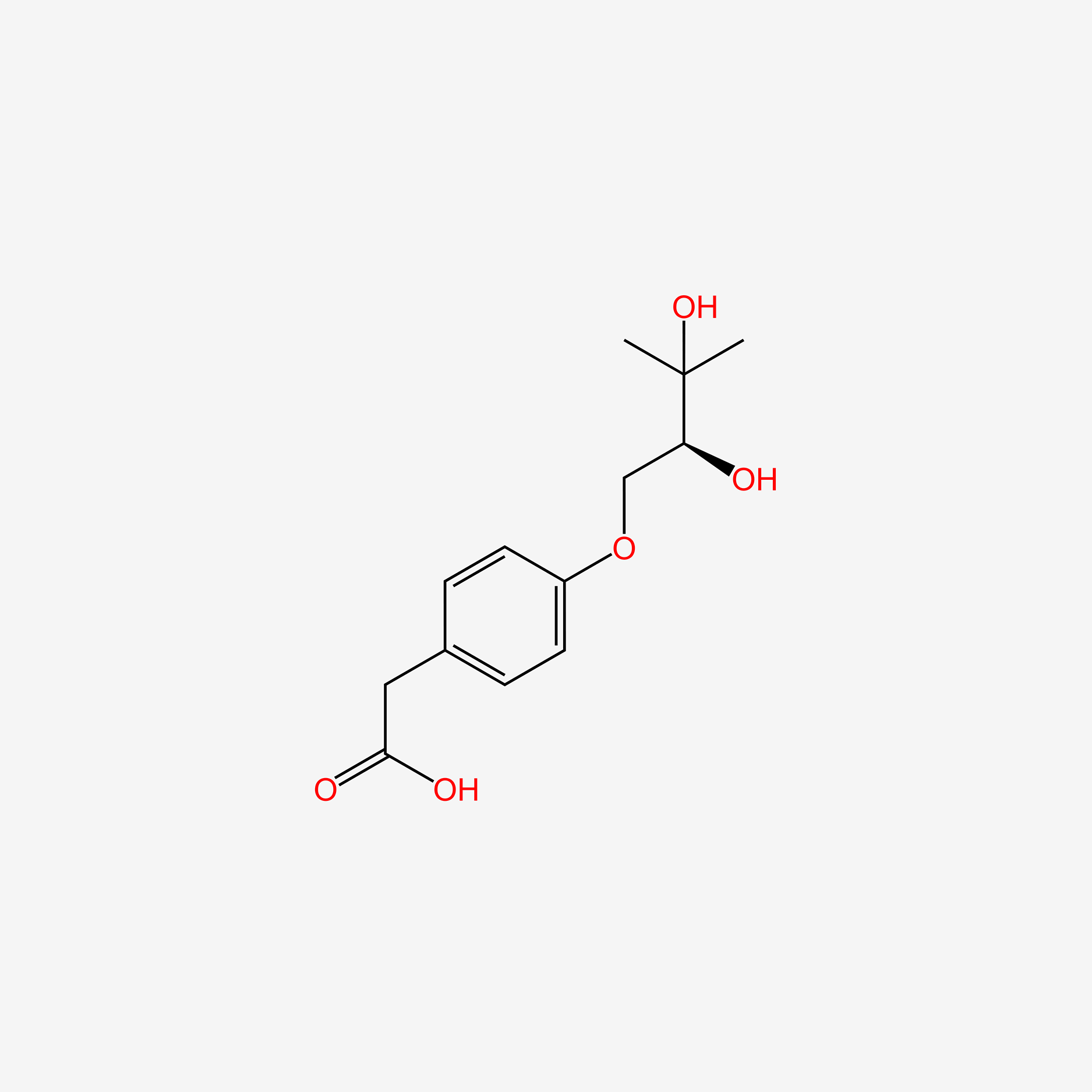 |
0.163 | D0K5CB | 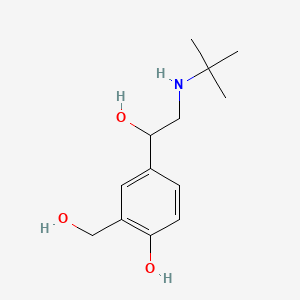 |
0.238 | ||
| ENC003124 | 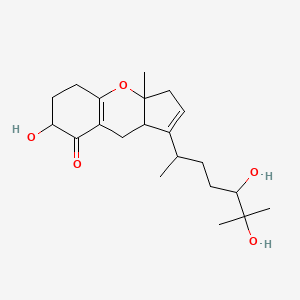 |
0.162 | D02ZJI | 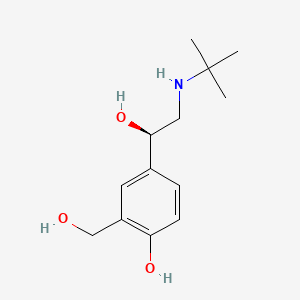 |
0.238 | ||