NPs Basic Information

|
Name |
Dihydromyrcenol
|
| Molecular Formula | C10H20O | |
| IUPAC Name* |
2,6-dimethyloct-7-en-2-ol
|
|
| SMILES |
CC(CCCC(C)(C)O)C=C
|
|
| InChI |
InChI=1S/C10H20O/c1-5-9(2)7-6-8-10(3,4)11/h5,9,11H,1,6-8H2,2-4H3
|
|
| InChIKey |
XSNQECSCDATQEL-UHFFFAOYSA-N
|
|
| Synonyms |
Dihydromyrcenol; 18479-58-8; 2,6-Dimethyloct-7-en-2-ol; 2,6-Dimethyl-7-octen-2-ol; 7-OCTEN-2-OL, 2,6-DIMETHYL-; 1,1,5-trimethyl-6-heptenol; Dihydro-|A; 3,7-Dimethyl-1-octen-7-ol; CHEBI:87528; 46L1B02ND9; DSSTox_CID_9317; BRN 1840872; EINECS 242-362-4; EINECS 246-787-6; UNII-46L1B02ND9; (1)-2,6-Dimethyloct-7-en-2-ol; Dihydromyrcenol, 99%; Dihydromyrcenol, >=99%; Myrcenol, 6,10-dihydro; EC 242-362-4; DSSTox_RID_78761; DSSTox_RID_79776; DSSTox_GSID_29317; DSSTox_GSID_41557; SCHEMBL29192; 2.6-dimethyl-7-octene-2-ol; CHEMBL3184487; DTXSID8029317; 2,6-Dimethyl-oct-7-en-2-ol; Dihydromyrcenol, analytical standard; Tox21_200132; Tox21_302391; MFCD00004474; AKOS015903381; NCGC00248536-01; NCGC00255603-01; NCGC00257686-01; AS-56700; CAS-18479-58-8; CAS-53219-21-9; CS-0204503; FT-0624964; FT-0640140; 2,6-DIMETHYL-7-OCTEN-2-OL [INCI]; E79376; EN300-366410; A812889; Q4465009; W-107790
|
|
| CAS | 18479-58-8 | |
| PubChem CID | 29096 | |
| ChEMBL ID | CHEMBL3184487 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 156.26 | ALogp: | 2.9 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 11 | QED Weighted: | 0.604 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.241 | MDCK Permeability: | 0.00002040 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.017 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.98 | Plasma Protein Binding (PPB): | 84.13% |
| Volume Distribution (VD): | 1.181 | Fu: | 22.01% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.1 | CYP1A2-substrate: | 0.818 |
| CYP2C19-inhibitor: | 0.105 | CYP2C19-substrate: | 0.864 |
| CYP2C9-inhibitor: | 0.062 | CYP2C9-substrate: | 0.853 |
| CYP2D6-inhibitor: | 0.011 | CYP2D6-substrate: | 0.797 |
| CYP3A4-inhibitor: | 0.1 | CYP3A4-substrate: | 0.269 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.968 | Half-life (T1/2): | 0.531 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.015 | Human Hepatotoxicity (H-HT): | 0.022 |
| Drug-inuced Liver Injury (DILI): | 0.026 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.042 | Maximum Recommended Daily Dose: | 0.032 |
| Skin Sensitization: | 0.109 | Carcinogencity: | 0.061 |
| Eye Corrosion: | 0.633 | Eye Irritation: | 0.987 |
| Respiratory Toxicity: | 0.02 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001263 | 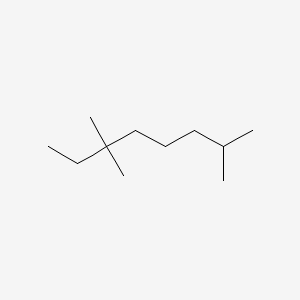 |
0.366 | D02VPX | 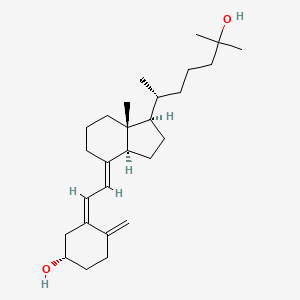 |
0.256 | ||
| ENC000906 | 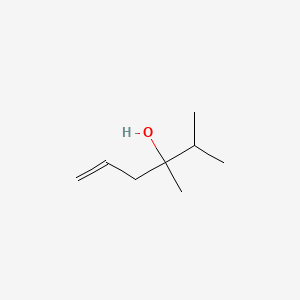 |
0.351 | D0T2PL | 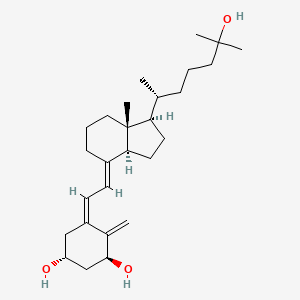 |
0.250 | ||
| ENC001239 | 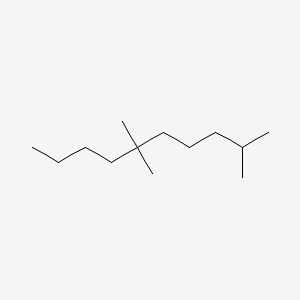 |
0.319 | D0R3QY |  |
0.214 | ||
| ENC000814 | 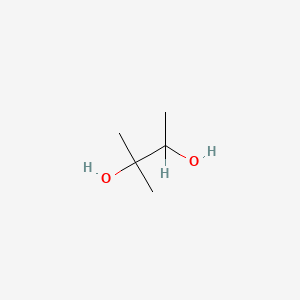 |
0.303 | D0M1PQ | 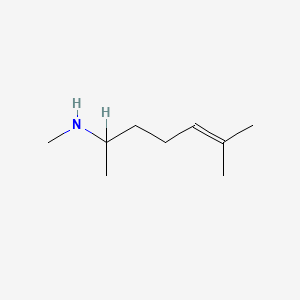 |
0.200 | ||
| ENC000529 | 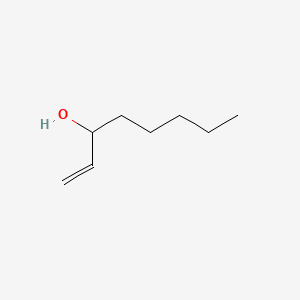 |
0.300 | D05VIX | 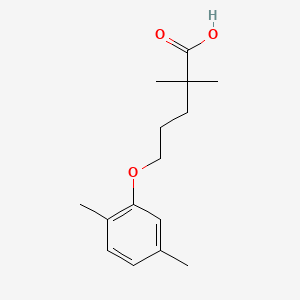 |
0.188 | ||
| ENC002570 | 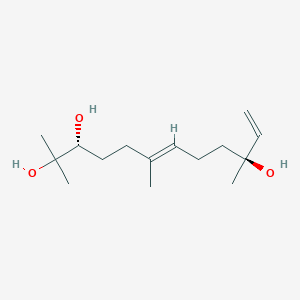 |
0.298 | D05BTM | 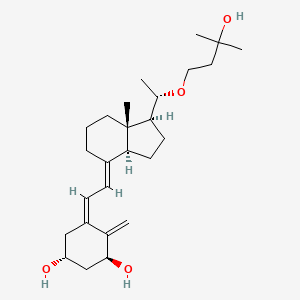 |
0.183 | ||
| ENC000629 | 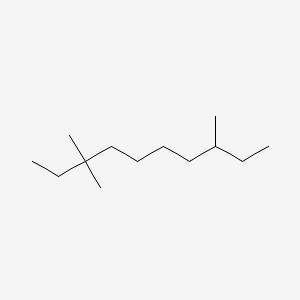 |
0.292 | D0D9NY |  |
0.182 | ||
| ENC001211 |  |
0.279 | D07SJT |  |
0.179 | ||
| ENC005621 |  |
0.279 | D00SJE | 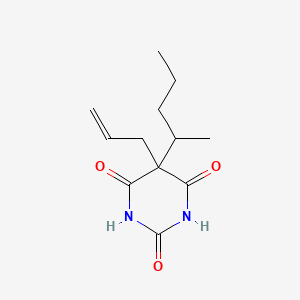 |
0.177 | ||
| ENC004079 | 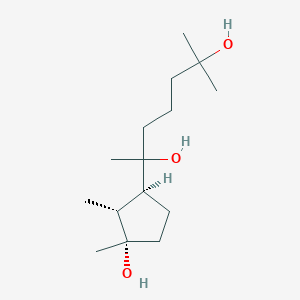 |
0.276 | D06NSA | 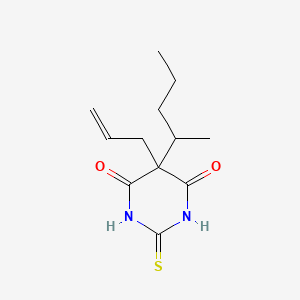 |
0.177 | ||