NPs Basic Information
|
Name |
1-Iodononane
|
| Molecular Formula | C9H19I | |
| IUPAC Name* |
1-iodononane
|
|
| SMILES |
CCCCCCCCCI
|
|
| InChI |
InChI=1S/C9H19I/c1-2-3-4-5-6-7-8-9-10/h2-9H2,1H3
|
|
| InChIKey |
OGSJMFCWOUHXHN-UHFFFAOYSA-N
|
|
| Synonyms |
1-Iodononane; 4282-42-2; Nonyl iodide; n-Nonyl iodide; Nonane, 1-iodo-; 1-n-Nonyl iodide; V528UR3HZE; NSC-5520; 1-Iodononane (Stabilized with Copper); IODONONANE; 1-iodo-n-nonane; Nonane,1-iodo-; NSC 5520; EINECS 224-286-3; 1-Iodononane, 95%; UNII-V528UR3HZE; DSSTox_CID_29157; DSSTox_RID_83376; DSSTox_GSID_49301; SCHEMBL873434; CHEMBL3184063; DTXSID6049301; NSC5520; Nonyl iodide (stabilzed over Cu); 1-Iodononane (stabilized over Cu); ZINC1686992; Tox21_202833; BBL011365; MFCD00001107; STL146462; AKOS005720905; NCGC00260379-01; AS-56404; CAS-4282-42-2; CS-0179369; FT-0607966; I0493; EN300-19869; D91171; A826017; J-802258; 6683-07-4
|
|
| CAS | 4282-42-2 | |
| PubChem CID | 20275 | |
| ChEMBL ID | CHEMBL3184063 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 254.15 | ALogp: | 5.8 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.349 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.442 | MDCK Permeability: | 0.00001410 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.369 |
| 30% Bioavailability (F30%): | 0.956 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.845 | Plasma Protein Binding (PPB): | 96.02% |
| Volume Distribution (VD): | 2.117 | Fu: | 5.18% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.95 | CYP1A2-substrate: | 0.55 |
| CYP2C19-inhibitor: | 0.613 | CYP2C19-substrate: | 0.243 |
| CYP2C9-inhibitor: | 0.33 | CYP2C9-substrate: | 0.9 |
| CYP2D6-inhibitor: | 0.081 | CYP2D6-substrate: | 0.239 |
| CYP3A4-inhibitor: | 0.11 | CYP3A4-substrate: | 0.11 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.212 | Half-life (T1/2): | 0.213 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.081 | Human Hepatotoxicity (H-HT): | 0.096 |
| Drug-inuced Liver Injury (DILI): | 0.386 | AMES Toxicity: | 0.055 |
| Rat Oral Acute Toxicity: | 0.133 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.791 | Carcinogencity: | 0.188 |
| Eye Corrosion: | 0.994 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.946 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
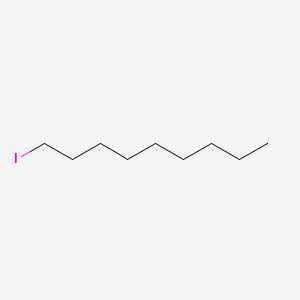
| ENC000502 | 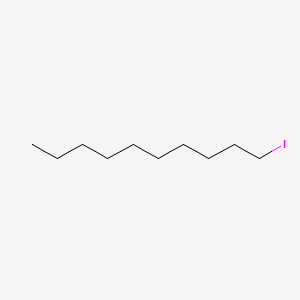 |
0.903 | D05ATI |  |
0.442 | ||
| ENC001240 | 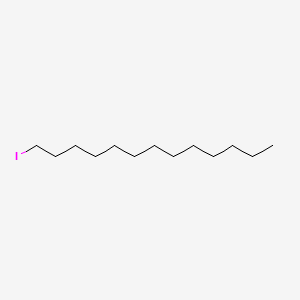 |
0.700 | D0Z5BC |  |
0.391 | ||
| ENC000330 |  |
0.697 | D0Z5SM |  |
0.390 | ||
| ENC000317 | 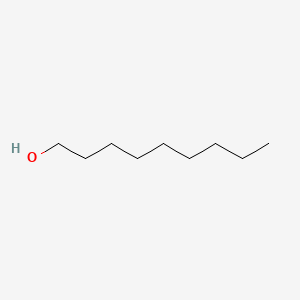 |
0.697 | D0Y8DP | 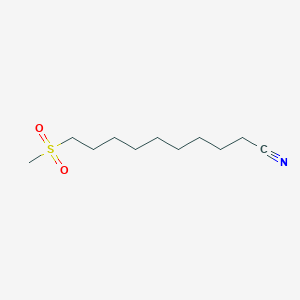 |
0.380 | ||
| ENC000493 | 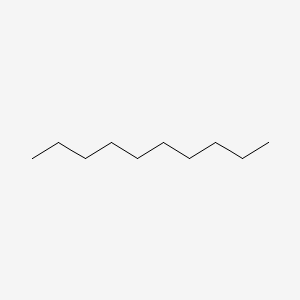 |
0.697 | D03ZJE | 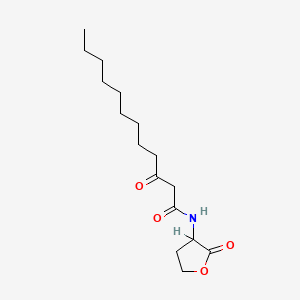 |
0.359 | ||
| ENC001237 | 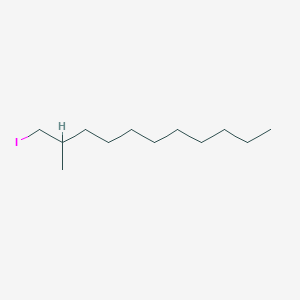 |
0.641 | D07ILQ |  |
0.354 | ||
| ENC000455 | 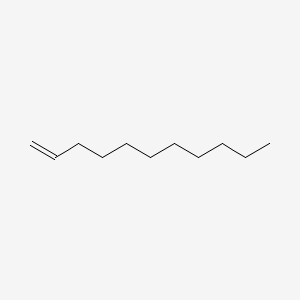 |
0.639 | D0XN8C |  |
0.338 | ||
| ENC000473 |  |
0.639 | D05QNO | 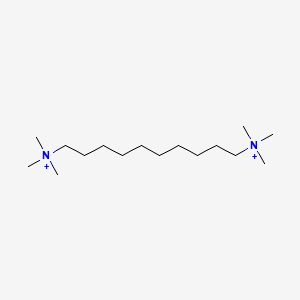 |
0.333 | ||
| ENC000267 | 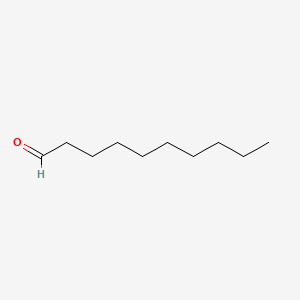 |
0.639 | D0O1PH |  |
0.324 | ||
| ENC000720 | 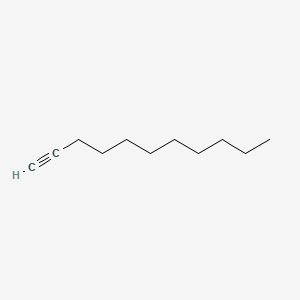 |
0.639 | D0AY9Q | 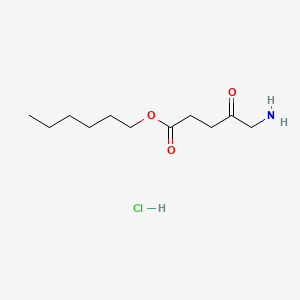 |
0.321 | ||