NPs Basic Information
|
Name |
Decane
|
| Molecular Formula | C10H22 | |
| IUPAC Name* |
decane
|
|
| SMILES |
CCCCCCCCCC
|
|
| InChI |
InChI=1S/C10H22/c1-3-5-7-9-10-8-6-4-2/h3-10H2,1-2H3
|
|
| InChIKey |
DIOQZVSQGTUSAI-UHFFFAOYSA-N
|
|
| Synonyms |
DECANE; n-Decane; 124-18-5; Nonane, methyl-; NK85062OIY; CHEBI:41808; NSC-8781; Decyl hydride; DSSTox_CID_4913; Decane, analytical standard; DSSTox_RID_77577; DSSTox_GSID_24913; CAS-124-18-5; D10; HSDB 63; CCRIS 653; NSC 8781; EINECS 204-686-4; UN2247; BRN 1696981; decan; UNII-NK85062OIY; normal-decane; AI3-24107; Decane, n-; MFCD00008954; Decane, 99%; DECANE [HSDB]; DECANE [INCI]; Decane, >=95%; SYNTSOL LP 10; EC 204-686-4; 4-01-00-00464 (Beilstein Handbook Reference); 73138-29-1; Decane, anhydrous, >=99%; CHEMBL134537; QSPL 111; WLN: 10H; DTXSID6024913; n-C10H22; NSC8781; Decane, ReagentPlus(R), >=99%; ZINC1648227; CACTUS NORMAL PARAFFIN N 10; Tox21_201881; Tox21_300336; LMFA11000568; STL280316; Decane, purum, >=95.0% (GC); Decane, purum, >=98.0% (GC); AKOS005145676; n-Decane 1000 microg/mL in Methanol; UN 2247; s11595; Decane, SAJ special grade, >=99.0%; NCGC00247996-01; NCGC00247996-02; NCGC00254283-01; NCGC00259430-01; 63335-87-5; LS-13903; n-Decane [UN2247] [Flammable liquid]; DB-089700; D0011; FT-0697465; S0282; S0554; EN300-19466; Q150717; J-005051; J-520211; F1908-0171; DBF497D1-4529-4457-841E-9D33CDF22B1C; 116372-01-1
|
|
| CAS | 124-18-5 | |
| PubChem CID | 15600 | |
| ChEMBL ID | CHEMBL134537 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 142.28 | ALogp: | 5.0 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.45 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.402 | MDCK Permeability: | 0.00001330 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.781 |
| 30% Bioavailability (F30%): | 0.993 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.808 | Plasma Protein Binding (PPB): | 97.14% |
| Volume Distribution (VD): | 3.073 | Fu: | 2.74% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.945 | CYP1A2-substrate: | 0.43 |
| CYP2C19-inhibitor: | 0.591 | CYP2C19-substrate: | 0.445 |
| CYP2C9-inhibitor: | 0.343 | CYP2C9-substrate: | 0.911 |
| CYP2D6-inhibitor: | 0.068 | CYP2D6-substrate: | 0.108 |
| CYP3A4-inhibitor: | 0.113 | CYP3A4-substrate: | 0.094 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.461 | Half-life (T1/2): | 0.221 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.1 | Human Hepatotoxicity (H-HT): | 0.013 |
| Drug-inuced Liver Injury (DILI): | 0.119 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.06 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.891 | Carcinogencity: | 0.058 |
| Eye Corrosion: | 0.993 | Eye Irritation: | 0.973 |
| Respiratory Toxicity: | 0.588 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000473 |  |
0.903 | D05ATI |  |
0.442 | ||
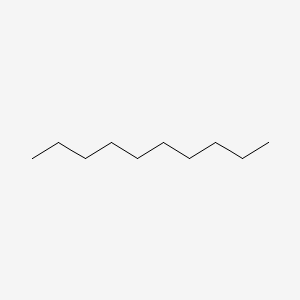
| ENC000261 | 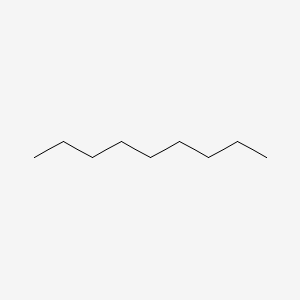 |
0.893 | D0Z5BC |  |
0.391 | ||
| ENC000272 | 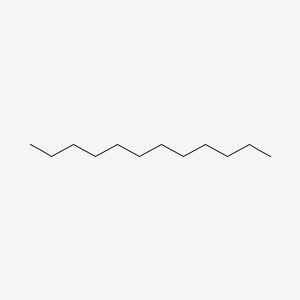 |
0.824 | D0Z5SM |  |
0.390 | ||
| ENC000421 |  |
0.757 | D0Y8DP | 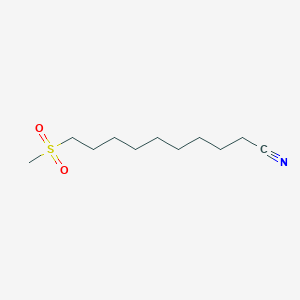 |
0.380 | ||
| ENC000422 | 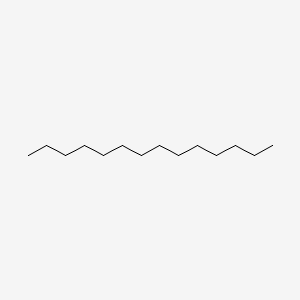 |
0.700 | D03ZJE | 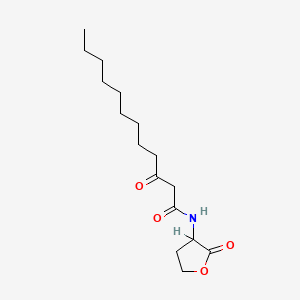 |
0.359 | ||
| ENC000330 | 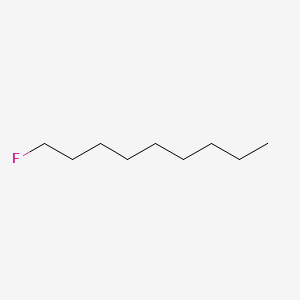 |
0.697 | D05QNO | 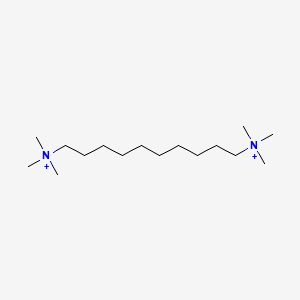 |
0.357 | ||
| ENC000317 | 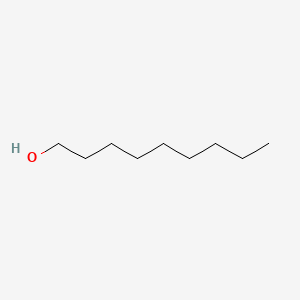 |
0.697 | D07ILQ |  |
0.354 | ||
| ENC000542 | 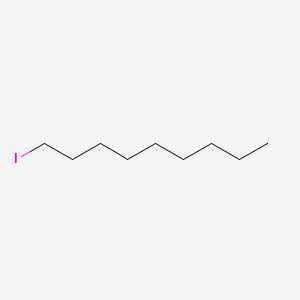 |
0.697 | D0XN8C |  |
0.338 | ||
| ENC000854 | 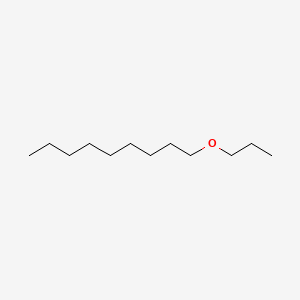 |
0.667 | D0O1PH |  |
0.324 | ||
| ENC000279 | 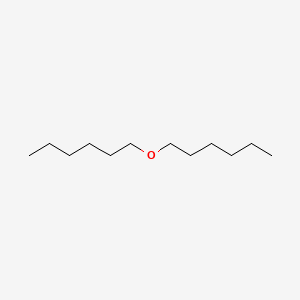 |
0.667 | D0AY9Q | 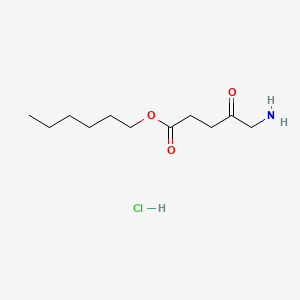 |
0.321 | ||