NPs Basic Information
|
Name |
Pentadecanoic acid
|
| Molecular Formula | C15H30O2 | |
| IUPAC Name* |
pentadecanoic acid
|
|
| SMILES |
CCCCCCCCCCCCCCC(=O)O
|
|
| InChI |
InChI=1S/C15H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15(16)17/h2-14H2,1H3,(H,16,17)
|
|
| InChIKey |
WQEPLUUGTLDZJY-UHFFFAOYSA-N
|
|
| Synonyms |
PENTADECANOIC ACID; 1002-84-2; Pentadecylic acid; n-Pentadecanoic acid; Pentadecyclic acid; n-Pentadecylic acid; C15:0; MFCD00002745; CCW02D961F; CHEMBL460025; CHEBI:42504; NSC-28486; NSC 28486; F15; EINECS 213-693-1; BRN 1773831; UNII-CCW02D961F; Pentadecyclate; n-Pentadecylate; AI3-36441; n-Pentadecanoate; pentadecanoic'acid; n-Pentadecanoicacid; pentadecansäure; tetradecylcarboxylic acid; tetradecyl carboxylic acid; Pentadecanoic acid, 99%; DSSTox_CID_1652; bmse000558; SCHEMBL6311; DSSTox_RID_76264; DSSTox_GSID_21652; Pentadecanoic acid, >=99%; 4-02-00-01147 (Beilstein Handbook Reference); WLN: QV14; DTXSID2021652; FA 15:0a; FEMA NO. 4334; AMY5935; PENTADECANOIC ACID [FHFI]; NSC28486; Tox21_201197; BDBM50242348; LMFA01010015; s6234; STL453783; ZINC30731069; AKOS015903762; CS-W004283; HY-W004283; Pentadecanoic acid, analytical standard; NCGC00248954-01; NCGC00258749-01; AS-57005; BP-27916; SY009985; CAS-1002-84-2; Pentadecanoic acid, ~99% (capillary GC); FT-0738251; P0035; EN300-176407; H10804; Pentadecanoic acid; n-Caprylic Acid Sodium Salt; 9B1C70C0-EF6E-4FCF-A3D3-DACCDCCF791C; A897509; Q418951; J-000083
|
|
| CAS | 1002-84-2 | |
| PubChem CID | 13849 | |
| ChEMBL ID | CHEMBL460025 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 242.4 | ALogp: | 5.8 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 13 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 17 | QED Weighted: | 0.435 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.996 | MDCK Permeability: | 0.00002670 |
| Pgp-inhibitor: | 0.017 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.7 |
| 30% Bioavailability (F30%): | 0.989 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.094 | Plasma Protein Binding (PPB): | 98.65% |
| Volume Distribution (VD): | 0.514 | Fu: | 1.12% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.298 | CYP1A2-substrate: | 0.199 |
| CYP2C19-inhibitor: | 0.146 | CYP2C19-substrate: | 0.154 |
| CYP2C9-inhibitor: | 0.213 | CYP2C9-substrate: | 0.988 |
| CYP2D6-inhibitor: | 0.007 | CYP2D6-substrate: | 0.06 |
| CYP3A4-inhibitor: | 0.02 | CYP3A4-substrate: | 0.021 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.343 | Half-life (T1/2): | 0.67 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.047 | Human Hepatotoxicity (H-HT): | 0.028 |
| Drug-inuced Liver Injury (DILI): | 0.043 | AMES Toxicity: | 0.005 |
| Rat Oral Acute Toxicity: | 0.033 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.869 | Carcinogencity: | 0.07 |
| Eye Corrosion: | 0.978 | Eye Irritation: | 0.981 |
| Respiratory Toxicity: | 0.873 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
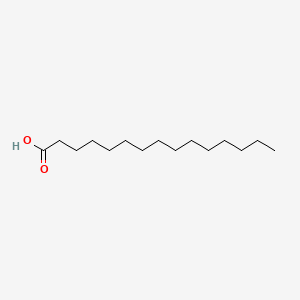
| ENC000050 | 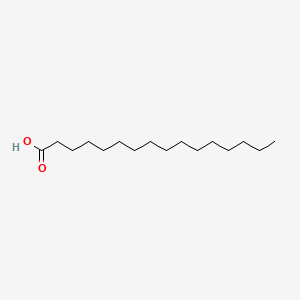 |
0.941 | D07ILQ |  |
0.688 | ||
| ENC000378 | 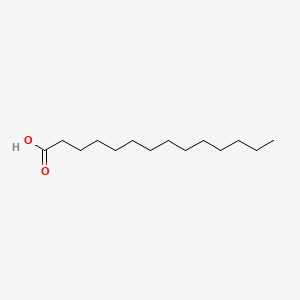 |
0.938 | D0O1PH |  |
0.629 | ||
| ENC000356 |  |
0.889 | D0Z5SM |  |
0.619 | ||
| ENC000110 | 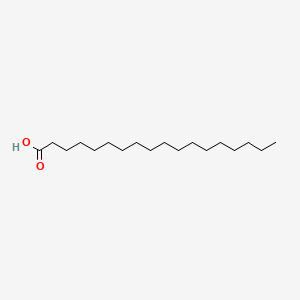 |
0.842 | D05ATI |  |
0.532 | ||
| ENC000102 | 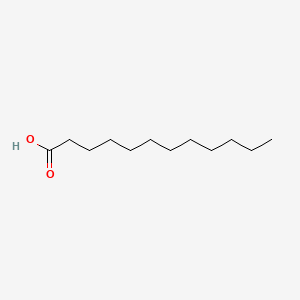 |
0.813 | D00AOJ |  |
0.513 | ||
| ENC000560 | 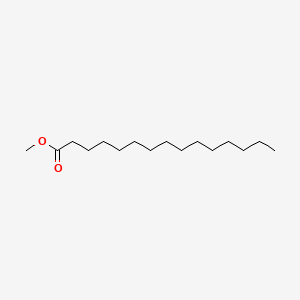 |
0.768 | D0XN8C |  |
0.507 | ||
| ENC000688 | 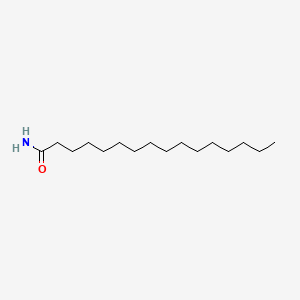 |
0.768 | D0Z5BC |  |
0.500 | ||
| ENC000357 | 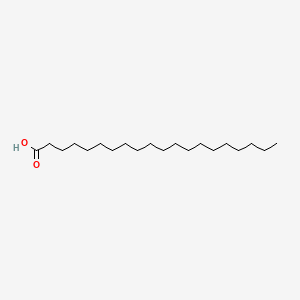 |
0.762 | D0P1RL | 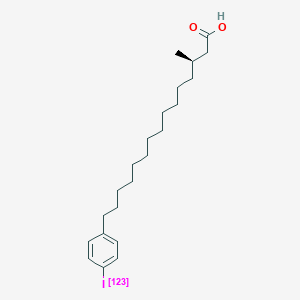 |
0.469 | ||
| ENC000642 | 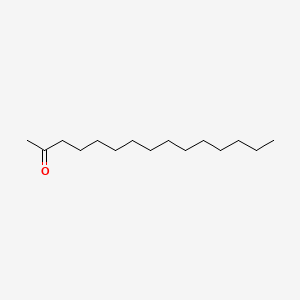 |
0.755 | D00FGR | 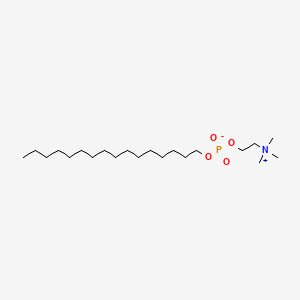 |
0.464 | ||
| ENC000270 | 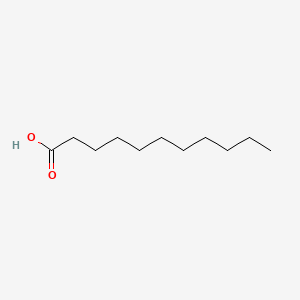 |
0.750 | D0O1TC | 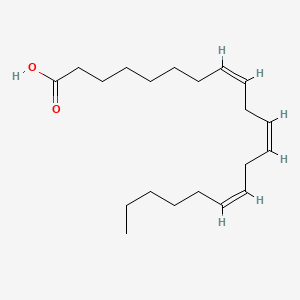 |
0.461 | ||