NPs Basic Information
|
Name |
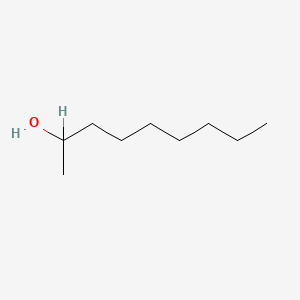
2-Nonanol
|
| Molecular Formula | C9H20O | |
| IUPAC Name* |
nonan-2-ol
|
|
| SMILES |
CCCCCCCC(C)O
|
|
| InChI |
InChI=1S/C9H20O/c1-3-4-5-6-7-8-9(2)10/h9-10H,3-8H2,1-2H3
|
|
| InChIKey |
NGDNVOAEIVQRFH-UHFFFAOYSA-N
|
|
| Synonyms |
2-NONANOL; NONAN-2-OL; 628-99-9; 1-Methyl-1-octanol; 2-Nonyl Alcohol; Methyl heptyl carbinol; Heptylmethylcarbinol; Heptyl methyl carbinol; Methylheptylcarbinol; n-Nonan-2-ol; FEMA No. 3315; Nonanol-(2); CHEBI:78304; NSC-9481; MFCD00004593; 292T5234DX; 2-hydroxynonane; 1-Octanol, methyl-; 2-Nonanol (natural); UNII-292T5234DX; NSC9481; NSC 9481; EINECS 211-065-1; 2-Nonanol, 99%; AI3-37210; DL-NONAN-2-OL; 2-NONANOL [FHFI]; 2-Nonanol, >=97%; SCHEMBL162308; CHEMBL454517; (+/-)-2-NONANOL; FEMA 3315; DTXSID60862323; LMFA05000619; AKOS009157271; SB83909; AS-56260; CS-0319684; FT-0626937; FT-0770586; FT-0771718; N0334; D97855; A868320; Q4596913
|
|
| CAS | 628-99-9 | |
| PubChem CID | 12367 | |
| ChEMBL ID | CHEMBL454517 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 144.25 | ALogp: | 3.4 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.565 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.279 | MDCK Permeability: | 0.00002310 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.246 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.841 |
| 30% Bioavailability (F30%): | 0.904 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.945 | Plasma Protein Binding (PPB): | 85.20% |
| Volume Distribution (VD): | 1.076 | Fu: | 19.87% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.846 | CYP1A2-substrate: | 0.801 |
| CYP2C19-inhibitor: | 0.32 | CYP2C19-substrate: | 0.561 |
| CYP2C9-inhibitor: | 0.216 | CYP2C9-substrate: | 0.918 |
| CYP2D6-inhibitor: | 0.013 | CYP2D6-substrate: | 0.254 |
| CYP3A4-inhibitor: | 0.027 | CYP3A4-substrate: | 0.131 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.088 | Half-life (T1/2): | 0.529 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.04 | Human Hepatotoxicity (H-HT): | 0.023 |
| Drug-inuced Liver Injury (DILI): | 0.04 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.029 | Maximum Recommended Daily Dose: | 0.018 |
| Skin Sensitization: | 0.448 | Carcinogencity: | 0.078 |
| Eye Corrosion: | 0.948 | Eye Irritation: | 0.986 |
| Respiratory Toxicity: | 0.116 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000459 |  |
0.688 | D05ATI |  |
0.298 | ||
| ENC000797 |  |
0.629 | D0I4DQ |  |
0.297 | ||
| ENC000554 |  |
0.588 | D01QLH | 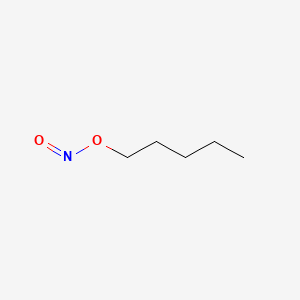 |
0.289 | ||
| ENC001148 | 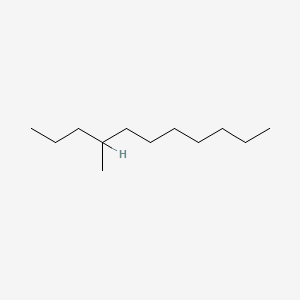 |
0.579 | D02MLW | 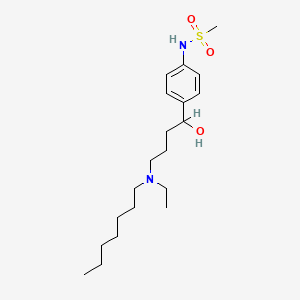 |
0.286 | ||
| ENC000558 | 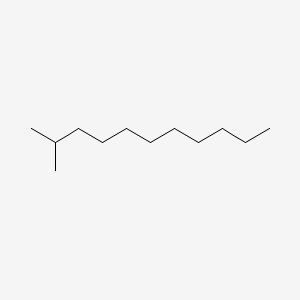 |
0.579 | D0AY9Q | 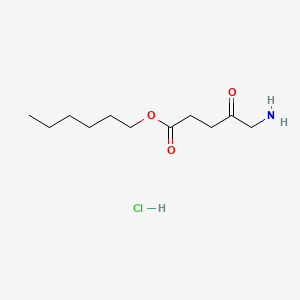 |
0.278 | ||
| ENC000583 | 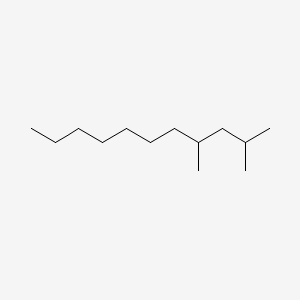 |
0.550 | D0G2KD | 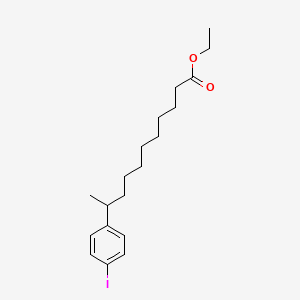 |
0.271 | ||
| ENC000398 | 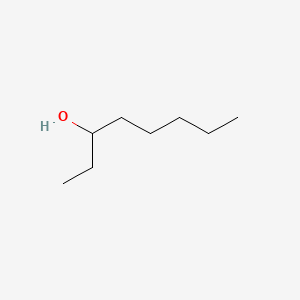 |
0.545 | D0D9NY | 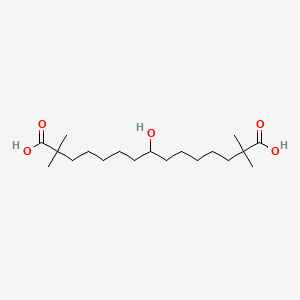 |
0.268 | ||
| ENC000519 | 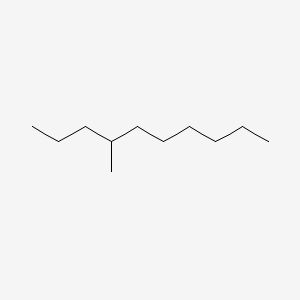 |
0.541 | D0Z5SM |  |
0.266 | ||
| ENC001155 | 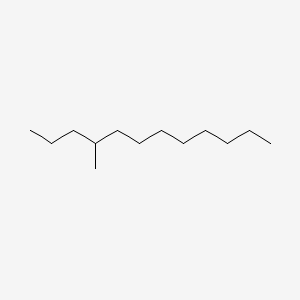 |
0.537 | D0E4WR | 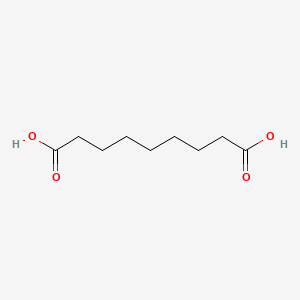 |
0.265 | ||
| ENC000490 |  |
0.537 | D0XN8C |  |
0.265 | ||