NPs Basic Information
|
Name |
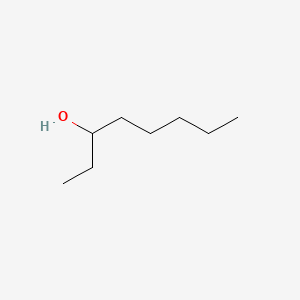
3-Octanol
|
| Molecular Formula | C8H18O | |
| IUPAC Name* |
octan-3-ol
|
|
| SMILES |
CCCCCC(CC)O
|
|
| InChI |
InChI=1S/C8H18O/c1-3-5-6-7-8(9)4-2/h8-9H,3-7H2,1-2H3
|
|
| InChIKey |
NMRPBPVERJPACX-UHFFFAOYSA-N
|
|
| Synonyms |
3-OCTANOL; Octan-3-ol; 589-98-0; 1-Ethylhexanol; Amyl ethyl carbinol; Ethyl-n-amylcarbinol; Ethylamylcarbinol; Octanol-3; Ethyl amyl carbinol; Amylethylcarbinol; D-n-Octanol; 20296-29-1; 3-Octyl Alcohol; dl-3-Octanol; n-Octan-3-ol; (S)-3-Octanol; FEMA No. 3581; Ethylhexyl alcohol; Ethyl pentyl carbinol; 73DZ0U3U1E; CHEBI:80945; 29063-28-3; 3-Octanol (natural); (1)-Octan-3-ol; EINECS 209-667-4; EINECS 243-713-4; EINECS 249-405-6; BRN 1697461; UNII-73DZ0U3U1E; AI3-37213; 1-ethyl-1-hexanol; octan-3(R,S)-ol; 3-Octanol, 97%; 3-Octanol, 99%; 3-OCTANOL [FCC]; 3-OCTANOL [FHFI]; OCTEN-3-OL-; 4-01-00-01756 (Beilstein Handbook Reference); SCHEMBL112339; 3-Octanol, analytical standard; CHEMBL487998; (+/-)-3-OCTANOL; DTXSID10862252; 3OL; AMY12157; 3-OCTANOL, (+/-)-; LMFA05000568; MFCD00004590; 3-Octanol, >=97%, FCC, FG; AKOS009156959; 3-Octanol, natural, >=97%, FCC, FG; CS-0152331; FT-0616280; FT-0616281; FT-0770830; O0121; n-Amyl ethyl carbinol Ethyl n-pentyl carbinol; C17144; D78240; EN300-7230130; A814407; A832101; Q27154917
|
|
| CAS | 589-98-0 | |
| PubChem CID | 11527 | |
| ChEMBL ID | CHEMBL487998 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 130.23 | ALogp: | 2.8 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 9 | QED Weighted: | 0.567 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.25 | MDCK Permeability: | 0.00002880 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.877 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.17 |
| 30% Bioavailability (F30%): | 0.229 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.963 | Plasma Protein Binding (PPB): | 66.48% |
| Volume Distribution (VD): | 1.226 | Fu: | 36.73% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.567 | CYP1A2-substrate: | 0.908 |
| CYP2C19-inhibitor: | 0.102 | CYP2C19-substrate: | 0.768 |
| CYP2C9-inhibitor: | 0.074 | CYP2C9-substrate: | 0.616 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.206 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.167 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.971 | Half-life (T1/2): | 0.663 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.027 | Human Hepatotoxicity (H-HT): | 0.024 |
| Drug-inuced Liver Injury (DILI): | 0.028 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.025 | Maximum Recommended Daily Dose: | 0.098 |
| Skin Sensitization: | 0.436 | Carcinogencity: | 0.085 |
| Eye Corrosion: | 0.953 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.327 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000554 | 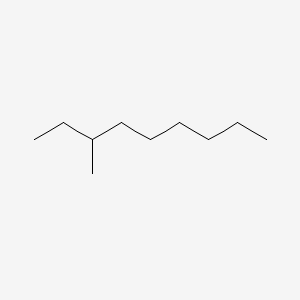 |
0.545 | D0Y3KG | 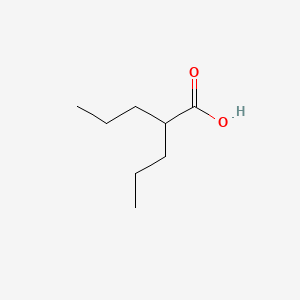 |
0.316 | ||
| ENC001211 | 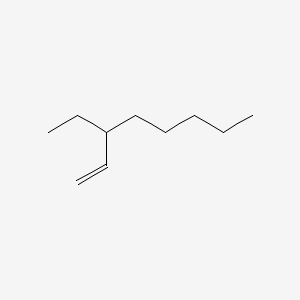 |
0.545 | D01QLH | 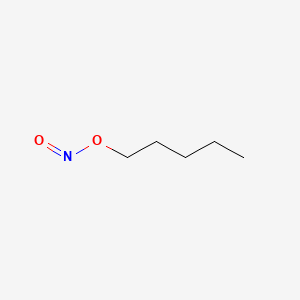 |
0.314 | ||
| ENC000420 | 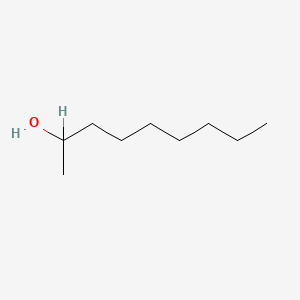 |
0.545 | D08SJZ | 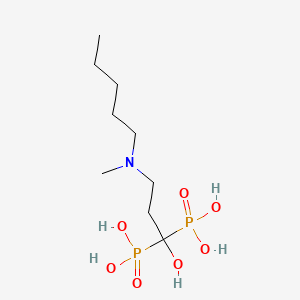 |
0.241 | ||
| ENC001899 | 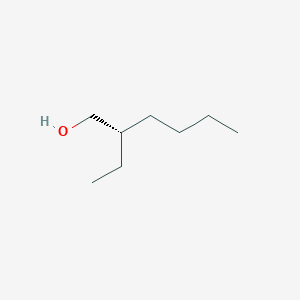 |
0.500 | D08QME | 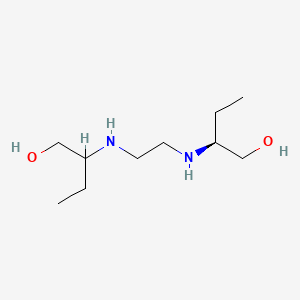 |
0.240 | ||
| ENC000529 | 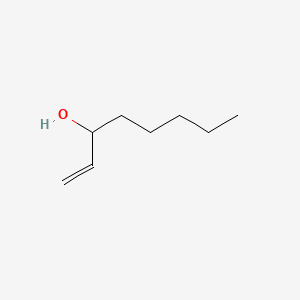 |
0.500 | D02MLW | 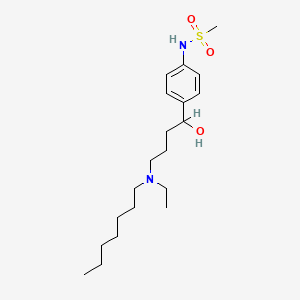 |
0.231 | ||
| ENC000580 | 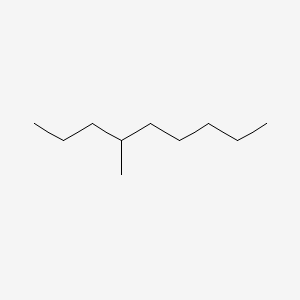 |
0.500 | D01WUA | 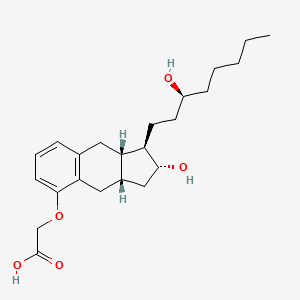 |
0.226 | ||
| ENC000220 | 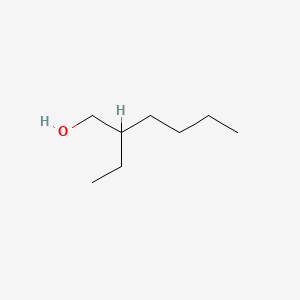 |
0.500 | D0D9NY | 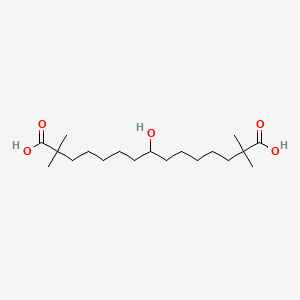 |
0.225 | ||
| ENC000797 | 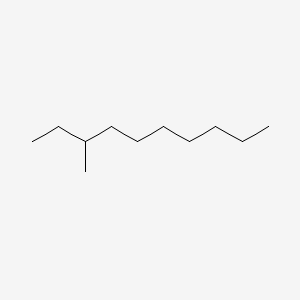 |
0.500 | D0I4DQ | 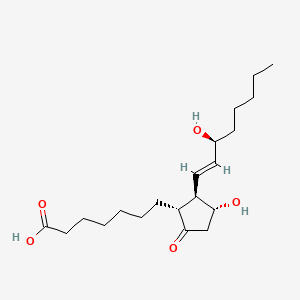 |
0.224 | ||
| ENC002444 | 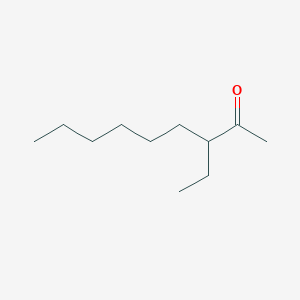 |
0.474 | D06FEA | 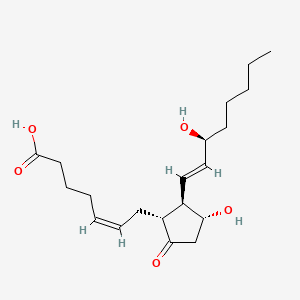 |
0.224 | ||
| ENC001126 | 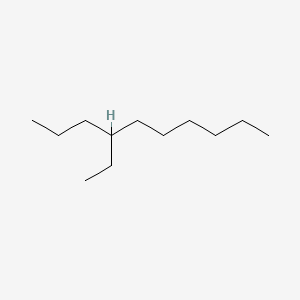 |
0.462 | D0AY9Q | 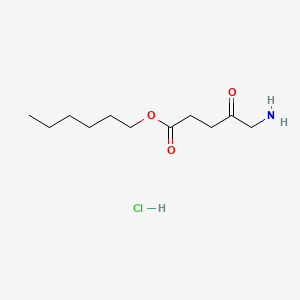 |
0.222 | ||