NPs Basic Information
|
Name |
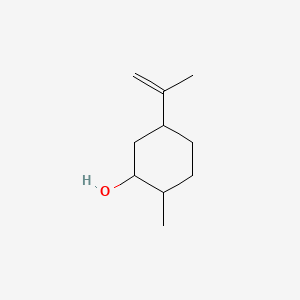
Dihydrocarveol
|
| Molecular Formula | C10H18O | |
| IUPAC Name* |
2-methyl-5-prop-1-en-2-ylcyclohexan-1-ol
|
|
| SMILES |
CC1CCC(CC1O)C(=C)C
|
|
| InChI |
InChI=1S/C10H18O/c1-7(2)9-5-4-8(3)10(11)6-9/h8-11H,1,4-6H2,2-3H3
|
|
| InChIKey |
KRCZYMFUWVJCLI-UHFFFAOYSA-N
|
|
| Synonyms |
Dihydrocarveol; 619-01-2; 8-p-Menthen-2-ol; 2-methyl-5-(prop-1-en-2-yl)cyclohexanol; 1,6-Dihydrocarveol; Neodihydrocarveol; Cyclohexanol, 2-methyl-5-(1-methylethenyl)-; 6-Methyl-3-isopropenylcyclohexanol; 2-methyl-5-prop-1-en-2-ylcyclohexan-1-ol; 2-Methyl-5-(1-methylethenyl)cyclohexanol; p-MENTH-8-EN-2-OL; Menth-8-en-2-ol; 5-Isopropenyl-2-methylcyclohexanol; 2-methyl-5-(prop-1-en-2-yl)cyclohexan-1-ol; CYCLOHEXANOL,2-METHYL-5-(1-METHYLETHENYL)-; FEMA No. 2379; EINECS 210-575-1; a dihydrocarveol; 1-Dihydrocarveol; Dihydro carveol neo; Neocarveol, dihydro-; SCHEMBL295511; GTPL6415; (1R,2S,5S)-neodihydrocarveol; CHEBI:50215; DTXSID60862303; 2-methyl-5-isopropenylcyclohexanol; AKOS015906522; SB84645; 2-Methyl-5-(1-propen-2-yl)cyclohexanol; DB-066237; DB-073026; FT-0624953; FT-0690209; FT-0771816; FT-0774461; p-Menth-8-en-2-ol, cis-1,2,trans-1,4-; C18017; Q1225152; 5-Isopropenyl-2-methylcyclohexanol, (1.alpha.,2.alpha.,5.beta.)-; Cyclohexanol, 2-methyl-5-(1-methylethenyl)-, (1R,2S,5S)-rel-; Cyclohexanol, 2-methyl-5-(1-methylethenyl)-, (1.alpha.,2.alpha.,5.beta.)-; N-Benzyl-2-[2-methyl-5-(4-methylphenyl)-2,3-dihydro-1,3,4-thiadiazol-2-yl]acetamide
|
|
| CAS | 619-01-2 | |
| PubChem CID | 12072 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 154.25 | ALogp: | 3.0 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.575 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.34 | MDCK Permeability: | 0.00001840 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.011 | 20% Bioavailability (F20%): | 0.017 |
| 30% Bioavailability (F30%): | 0.012 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.973 | Plasma Protein Binding (PPB): | 49.59% |
| Volume Distribution (VD): | 1.197 | Fu: | 42.73% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.134 | CYP1A2-substrate: | 0.685 |
| CYP2C19-inhibitor: | 0.024 | CYP2C19-substrate: | 0.839 |
| CYP2C9-inhibitor: | 0.024 | CYP2C9-substrate: | 0.804 |
| CYP2D6-inhibitor: | 0.003 | CYP2D6-substrate: | 0.879 |
| CYP3A4-inhibitor: | 0.02 | CYP3A4-substrate: | 0.296 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.601 | Half-life (T1/2): | 0.367 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.027 | Human Hepatotoxicity (H-HT): | 0.214 |
| Drug-inuced Liver Injury (DILI): | 0.045 | AMES Toxicity: | 0.026 |
| Rat Oral Acute Toxicity: | 0.039 | Maximum Recommended Daily Dose: | 0.065 |
| Skin Sensitization: | 0.146 | Carcinogencity: | 0.449 |
| Eye Corrosion: | 0.356 | Eye Irritation: | 0.945 |
| Respiratory Toxicity: | 0.718 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001888 | 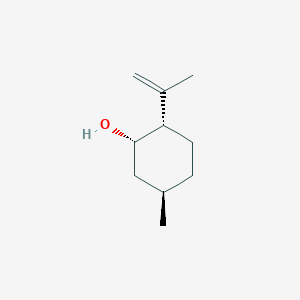 |
0.526 | D04CSZ | 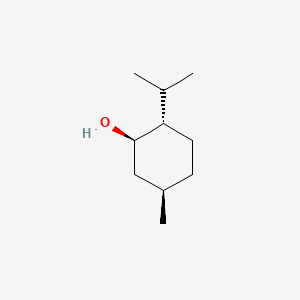 |
0.349 | ||
| ENC001284 | 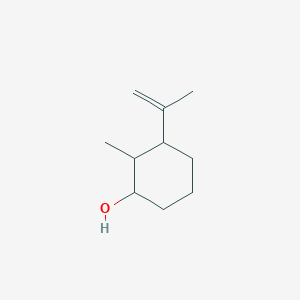 |
0.487 | D05HXX | 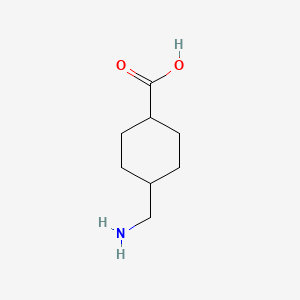 |
0.229 | ||
| ENC001295 | 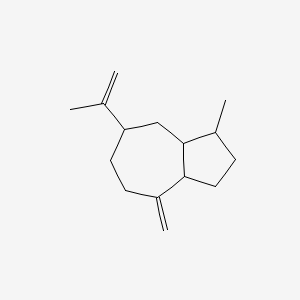 |
0.458 | D0N6FH | 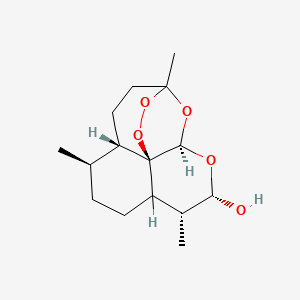 |
0.214 | ||
| ENC001816 | 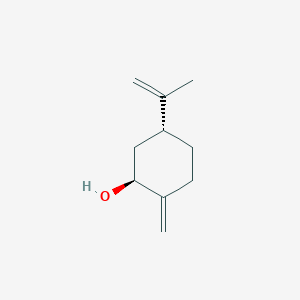 |
0.450 | D04SFH |  |
0.213 | ||
| ENC000567 |  |
0.450 | D0V8HA |  |
0.196 | ||
| ENC000808 |  |
0.429 | D00VZZ |  |
0.192 | ||
| ENC002124 |  |
0.396 | D04URO |  |
0.190 | ||
| ENC000839 |  |
0.373 | D0R7WU |  |
0.189 | ||
| ENC001619 | 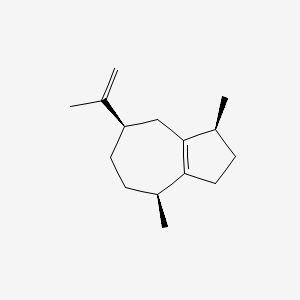 |
0.373 | D0I2SD | 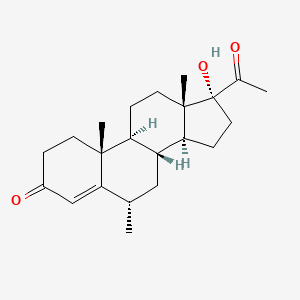 |
0.183 | ||
| ENC005497 | 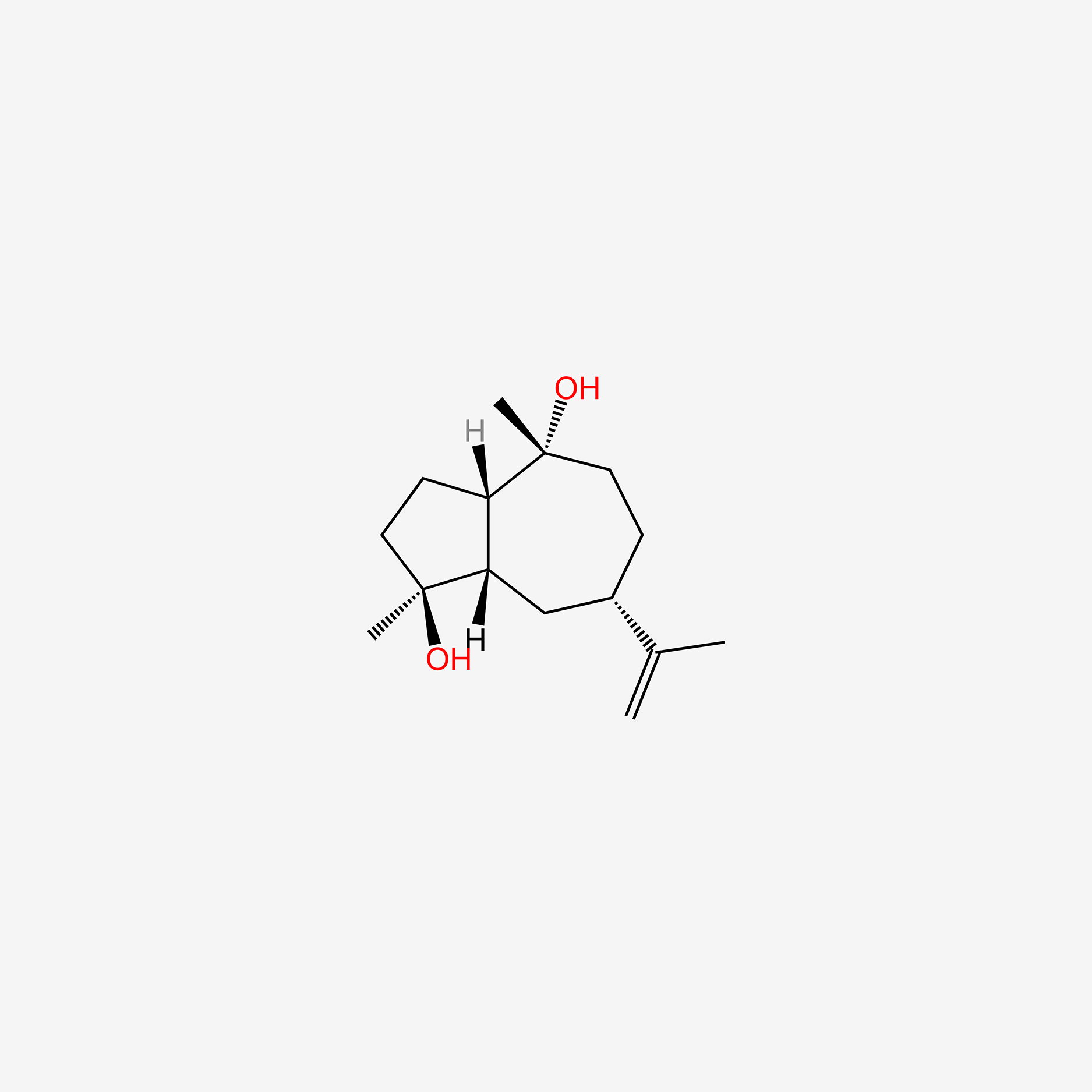 |
0.370 | D0S3WH | 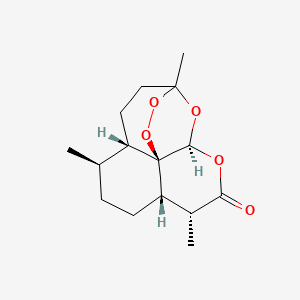 |
0.181 | ||