NPs Basic Information
|
Name |
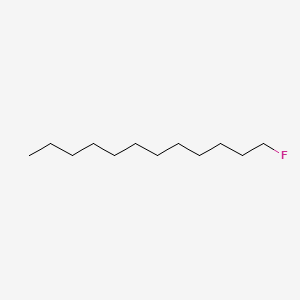
1-Fluorododecane
|
| Molecular Formula | C12H25F | |
| IUPAC Name* |
1-fluorododecane
|
|
| SMILES |
CCCCCCCCCCCCF
|
|
| InChI |
InChI=1S/C12H25F/c1-2-3-4-5-6-7-8-9-10-11-12-13/h2-12H2,1H3
|
|
| InChIKey |
YHYBNVZCQIDLSQ-UHFFFAOYSA-N
|
|
| Synonyms |
1-FLUORODODECANE; 334-68-9; Dodecyl fluoride; Dodecane, 1-fluoro-; n-Dodecyl fluoride; EINECS 206-381-1; BRN 1738884; 1-fluoro-dodecane; 1-fluoranyldodecane; 1-Fluorododecane, 98%; 4-01-00-00500 (Beilstein Handbook Reference); SCHEMBL318325; DTXSID6074706; ZINC2040783; MFCD00013580; AKOS007930497; AS-81226; DB-048416; FT-0607782; A821788; J-019203
|
|
| CAS | 334-68-9 | |
| PubChem CID | 9548 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 188.32 | ALogp: | 6.2 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 10 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 13 | QED Weighted: | 0.41 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.708 | MDCK Permeability: | 0.00002370 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.884 |
| 30% Bioavailability (F30%): | 0.966 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.367 | Plasma Protein Binding (PPB): | 97.59% |
| Volume Distribution (VD): | 3.383 | Fu: | 1.85% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.67 | CYP1A2-substrate: | 0.231 |
| CYP2C19-inhibitor: | 0.484 | CYP2C19-substrate: | 0.094 |
| CYP2C9-inhibitor: | 0.209 | CYP2C9-substrate: | 0.913 |
| CYP2D6-inhibitor: | 0.11 | CYP2D6-substrate: | 0.186 |
| CYP3A4-inhibitor: | 0.162 | CYP3A4-substrate: | 0.08 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.827 | Half-life (T1/2): | 0.109 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.21 |
| Drug-inuced Liver Injury (DILI): | 0.072 | AMES Toxicity: | 0.035 |
| Rat Oral Acute Toxicity: | 0.84 | Maximum Recommended Daily Dose: | 0.289 |
| Skin Sensitization: | 0.621 | Carcinogencity: | 0.617 |
| Eye Corrosion: | 0.983 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.982 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000739 |  |
0.860 | D05ATI |  |
0.615 | ||
| ENC000421 |  |
0.762 | D0Z5SM |  |
0.542 | ||
| ENC000276 |  |
0.762 | D07ILQ |  |
0.492 | ||
| ENC000330 |  |
0.757 | D00AOJ |  |
0.444 | ||
| ENC000422 |  |
0.711 | D0O1PH |  |
0.431 | ||
| ENC000475 |  |
0.711 | D05QNO |  |
0.417 | ||
| ENC001240 | 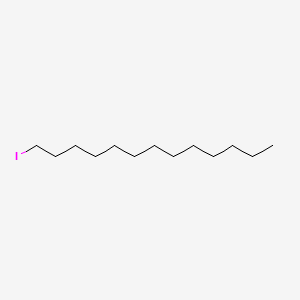 |
0.711 | D00FGR | 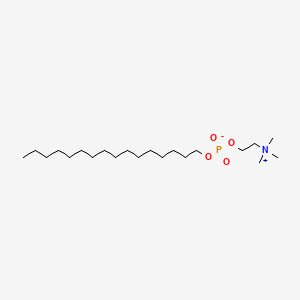 |
0.400 | ||
| ENC000274 | 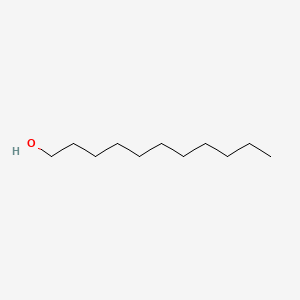 |
0.690 | D0Y8DP | 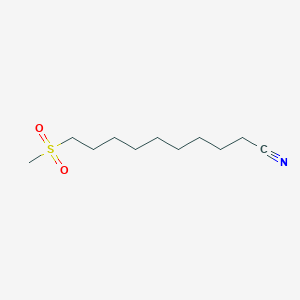 |
0.393 | ||
| ENC000272 |  |
0.690 | D0XN8C |  |
0.371 | ||
| ENC000266 |  |
0.681 | D0P1RL |  |
0.350 | ||