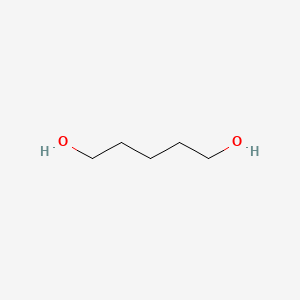
NPs Basic Information
|
Name |
1,5-Pentanediol
|
| Molecular Formula | C5H12O2 | |
| IUPAC Name* |
pentane-1,5-diol
|
|
| SMILES |
C(CCO)CCO
|
|
| InChI |
InChI=1S/C5H12O2/c6-4-2-1-3-5-7/h6-7H,1-5H2
|
|
| InChIKey |
ALQSHHUCVQOPAS-UHFFFAOYSA-N
|
|
| Synonyms |
1,5-PENTANEDIOL; Pentane-1,5-diol; 111-29-5; 1,5-Dihydroxypentane; Pentamethylene glycol; 1,5-Pentylene glycol; 1,5 Pentanediol; 1,5-Pentamethylene glycol; NSC 5927; 1,5-Pentandiol; .alpha.,.omega.-Pentanediol; 07UXZ0SCST; NSC-5927; 31784-47-1; alpha,omega-Pentanediol; EINECS 203-854-4; UNII-07UXZ0SCST; BRN 1560130; AI3-03318; Pentylene Gylcol; 1.5-pentanediol; 9JE; pentan-1,5-diol; 1,5-pentane diol; .omega.-Pentanediol; MFCD00002978; Pentane diol-1,5; EC 203-854-4; 1,5-Pentanediol, 96%; WLN: Q5Q; DSSTox_CID_21256; DSSTox_RID_79666; HO(CH2)5OH; DSSTox_GSID_41256; SCHEMBL18788; 4-01-00-02540 (Beilstein Handbook Reference); CHEMBL448289; 1,5-PENTANEDIOL [MI]; DTXSID2041256; HSDB 6807; 1,5-PENTANEDIOL [INCI]; NSC5927; CHEBI:185431; ZINC1687319; Tox21_300880; AKOS009158215; CS-W020635; NCGC00248201-01; NCGC00254784-01; BP-30035; CAS-111-29-5; FT-0606982; P0050; 1,5-Pentanediol, purum, >=95.0% (GC); 1,5-Pentanediol, purum, >=97.0% (GC); D77911; EN300-122591; H-1745; 1,5-Pentanediol, Vetec(TM) reagent grade, 96%; A802337; Q161557; J-002554; F0001-0238
|
|
| CAS | 111-29-5 | |
| PubChem CID | 8105 | |
| ChEMBL ID | CHEMBL448289 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 104.15 | ALogp: | -0.1 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 0 |
| Heavy Atoms: | 7 | QED Weighted: | 0.511 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.358 | MDCK Permeability: | 0.00010337 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.018 | 20% Bioavailability (F20%): | 0.125 |
| 30% Bioavailability (F30%): | 0.954 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.558 | Plasma Protein Binding (PPB): | 8.54% |
| Volume Distribution (VD): | 0.828 | Fu: | 86.12% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.045 | CYP1A2-substrate: | 0.308 |
| CYP2C19-inhibitor: | 0.018 | CYP2C19-substrate: | 0.145 |
| CYP2C9-inhibitor: | 0.005 | CYP2C9-substrate: | 0.111 |
| CYP2D6-inhibitor: | 0.002 | CYP2D6-substrate: | 0.08 |
| CYP3A4-inhibitor: | 0.004 | CYP3A4-substrate: | 0.125 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.789 | Half-life (T1/2): | 0.861 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.042 | Human Hepatotoxicity (H-HT): | 0.04 |
| Drug-inuced Liver Injury (DILI): | 0.031 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.025 | Maximum Recommended Daily Dose: | 0.01 |
| Skin Sensitization: | 0.639 | Carcinogencity: | 0.164 |
| Eye Corrosion: | 0.967 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.029 |