NPs Basic Information
|
Name |
1-Octanol
|
| Molecular Formula | C8H18O | |
| IUPAC Name* |
octan-1-ol
|
|
| SMILES |
CCCCCCCCO
|
|
| InChI |
InChI=1S/C8H18O/c1-2-3-4-5-6-7-8-9/h9H,2-8H2,1H3
|
|
| InChIKey |
KBPLFHHGFOOTCA-UHFFFAOYSA-N
|
|
| Synonyms |
1-octanol; Octan-1-ol; octanol; 111-87-5; N-octanol; Octyl alcohol; caprylic alcohol; Capryl alcohol; n-Octyl alcohol; Heptyl carbinol; 1-Hydroxyoctane; Primary octyl alcohol; Octilin; Alcohol C-8; n-Octan-1-ol; Alfol 8; Sipol L8; Lorol 20; Dytol M-83; n-Caprylic alcohol; 1-Octyl alcohol; n-Heptyl carbinol; EPAL 8; octyl-alcohol; N-octyl-alcohol; Octyl alcohol, normal-primary; Lorol C 8-98; FEMA No. 2800; n-Capryl alcohol; NSC 9823; 1-Oktanol; Alcohol C8; 68603-15-6; Prim-n-octyl alcohol; Emery 3322; Emery 3324; MFCD00002988; CHEBI:16188; NSC-9823; NV1779205D; DSSTox_CID_1940; C8 alcohol; DSSTox_RID_76416; DSSTox_GSID_21940; Octyl alcohol, primary; Caswell No. 611A; Octyl alcohol (natural); FEMA Number 2800; CAS-111-87-5; OC9; CCRIS 9099; HSDB 700; Octanol (all isomers); EINECS 203-917-6; EPA Pesticide Chemical Code 079037; octylalcohol; caprylyl alcohol; AI3-02169; UNII-NV1779205D; 1-n-octanol; 2-Capryl alcohol; Lorol C8; Octanol-(1); ALFOL 8 ALCOHOL; 2-Octanol ~99%; bmse000970; bmse000980; 1-OCTANOL [FHFI]; 1-OCTANOL [HSDB]; 1-OCTANOL [MI]; EC 203-917-6; Octan-2-ol 98+ %; SCHEMBL8822; Octyl alcohol normal-primary; OCTYL ALCOHOL [FCC]; KALCOHL-0898; WLN: Q8; MLS001055318; CHEMBL26215; NACOL 8-99 ALCOHOL; 1-Octanol, analytical standard; GTPL4278; CAPRYLYL ALCOHOL [INCI]; DTXSID7021940; N-OCTYL ALCOHOL, PRIMARY; BDBM22606; 1-Octanol, anhydrous, >=99%; NSC9823; HMS3039O07; 1-Octanol, for HPLC, >=99%; ZINC1532735; 1-Octanol, ACS reagent, >=99%; 1-Octanol, ReagentPlus(R), 99%; Tox21_201373; Tox21_300096; c0045; LMFA05000130; STL264193; 1-Octanol, >=98%, FCC, FG; 1-Octanol, natural, >=98%, FCC; AKOS000120100; DB12452; HY-W032013; NCGC00091003-01; NCGC00091003-02; NCGC00091003-03; NCGC00091003-04; NCGC00091003-05; NCGC00254099-01; NCGC00258924-01; BP-21329; LS-13539; SMR000673567; 1-Octanol, puriss., >=99.5% (GC); 1-Octanol, SAJ first grade, >=75.0%; 1-Octanol, JIS special grade, >=98.0%; 1-Octanol, Vetec(TM) reagent grade, 98%; CS-0076037; FT-0608179; O0036; O0212; EN300-19311; C00756; 1-Octanol, ACS spectrophotometric grade, >=99%; Q161666; J-002650; F0001-0248; Z104473500; 958E4752-AAC3-4F72-A0BF-02D95F9E8071; 67700-96-3
|
|
| CAS | 111-87-5 | |
| PubChem CID | 957 | |
| ChEMBL ID | CHEMBL26215 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 130.23 | ALogp: | 3.0 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 9 | QED Weighted: | 0.548 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.264 | MDCK Permeability: | 0.00002450 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.033 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.261 |
| 30% Bioavailability (F30%): | 0.956 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.986 | Plasma Protein Binding (PPB): | 79.31% |
| Volume Distribution (VD): | 1.048 | Fu: | 27.59% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.852 | CYP1A2-substrate: | 0.626 |
| CYP2C19-inhibitor: | 0.185 | CYP2C19-substrate: | 0.17 |
| CYP2C9-inhibitor: | 0.148 | CYP2C9-substrate: | 0.68 |
| CYP2D6-inhibitor: | 0.007 | CYP2D6-substrate: | 0.104 |
| CYP3A4-inhibitor: | 0.022 | CYP3A4-substrate: | 0.104 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.609 | Half-life (T1/2): | 0.656 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.06 | Human Hepatotoxicity (H-HT): | 0.02 |
| Drug-inuced Liver Injury (DILI): | 0.036 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.077 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.844 | Carcinogencity: | 0.124 |
| Eye Corrosion: | 0.989 | Eye Irritation: | 0.985 |
| Respiratory Toxicity: | 0.177 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
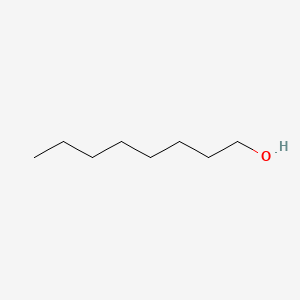
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000317 | 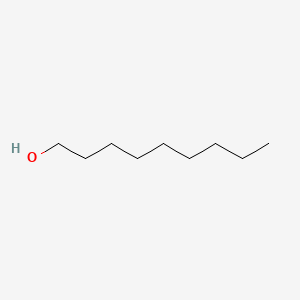 |
0.893 | D05ATI |  |
0.385 | ||
| ENC000274 | 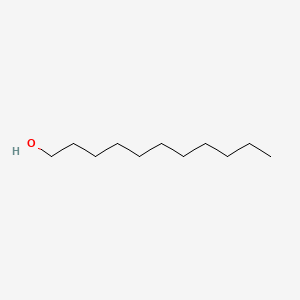 |
0.735 | D00AOJ |  |
0.373 | ||
| ENC000276 |  |
0.676 | D07ILQ |  |
0.371 | ||
| ENC000261 | 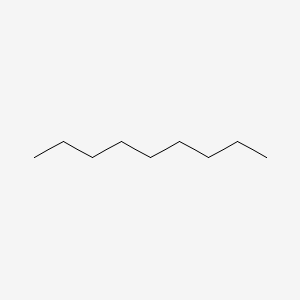 |
0.667 | D0E4WR | 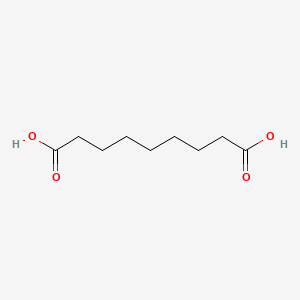 |
0.364 | ||
| ENC000139 | 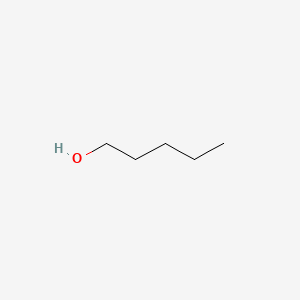 |
0.640 | D0Z5BC |  |
0.356 | ||
| ENC000263 | 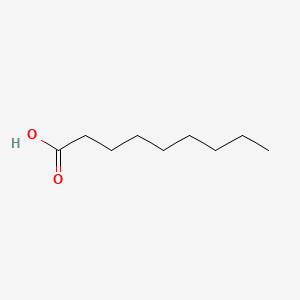 |
0.618 | D0Z5SM |  |
0.339 | ||
| ENC000606 | 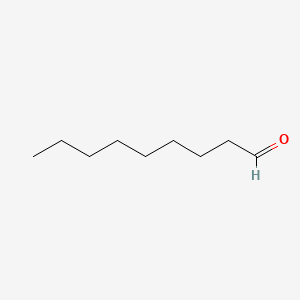 |
0.606 | D07UHS |  |
0.338 | ||
| ENC000460 | 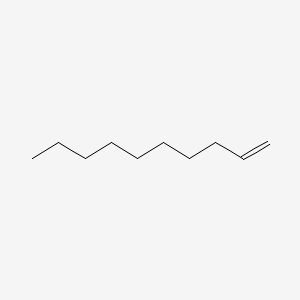 |
0.606 | D0O1PH |  |
0.338 | ||
| ENC000542 | 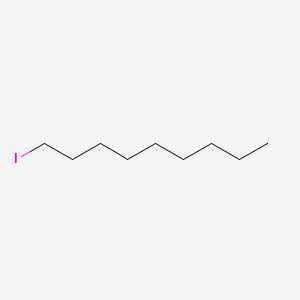 |
0.606 | D0XN8C |  |
0.333 | ||
| ENC000330 | 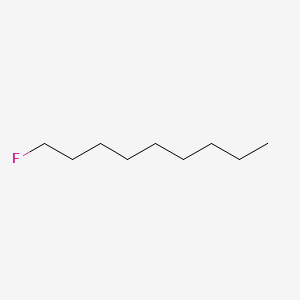 |
0.606 | D0Y8DP |  |
0.320 | ||