NPs Basic Information
|
Name |
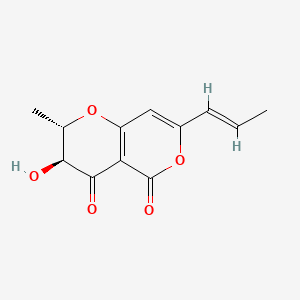
Radicinin
|
| Molecular Formula | C12H12O5 | |
| IUPAC Name* |
(2S,3S)-3-hydroxy-2-methyl-7-[(E)-prop-1-enyl]-2,3-dihydropyrano[3,2-c]pyran-4,5-dione
|
|
| SMILES |
C/C=C/C1=CC2=C(C(=O)[C@H]([C@@H](O2)C)O)C(=O)O1
|
|
| InChI |
InChI=1S/C12H12O5/c1-3-4-7-5-8-9(12(15)17-7)11(14)10(13)6(2)16-8/h3-6,10,13H,1-2H3/b4-3+/t6-,10-/m0/s1
|
|
| InChIKey |
SDKXGAICTNHFCN-DCJAWTJCSA-N
|
|
| Synonyms |
Radicinin; Stemphylone; 10088-95-6; NSC118343; G01Z2N575M; (2S,3S)-3-hydroxy-2-methyl-7-[(E)-prop-1-enyl]-2,3-dihydropyrano[3,2-c]pyran-4,5-dione; NSC-118343; 1402-20-6; Radicinin (VAN); MLS002706791; NSC 118343; UNII-G01Z2N575M; CCRIS 8483; RADICININ [MI]; 2,3-Dihydro-3-alpha-hydroxy-2-beta-methyl-7-propenyl-4H,5H-pyrano(4,3-b)pyran-4,5-dione; SCHEMBL1531040; SCHEMBL1531042; CHEMBL1994984; DTXSID70871946; CHEBI:183741; ZINC101123832; 4H,5H-Pyrano(4,3-b)pyran-4,5-dione, 2,3-dihydro-3-alpha-hydroxy-2-beta-methyl-7-propenyl-; 2,5H-pyrano[4,3-b]pyran-4,5-dione; HY-113816; CS-0062918; WLN: T66 BVO GO JV&TJ D1U2 H1 IQ; Q27278392; (2S,3S)-3-hydroxy-2-methyl-7-[(E)-prop-1-enyl]-2,3-dihydropyrano[4,3-b]pyran-4,5-dione; 4H,3-b]pyran-4,5-dione, 2,3-dihydro-3.alpha.-hydroxy-2.beta.-methyl-7-propenyl-; (2S,3S)-3-hydroxy-2-methyl-7-((E)-prop-1-en-1-yl)-2,3-dihydro-4H,5H-pyrano[4,3-b]pyran-4,5-dione; (2S,3S)-3-Hydroxy-2-methyl-7-[(1E)-prop-1-en-1-yl]-2,3-dihydro-4H,5H-pyrano[4,3-b]pyran-4,5-dione; 2H-PYRAN-5-CARBOXYLIC ACID, 3,4-DIHYDRO-3-HYDROXY-6-(2-HYDROXY-1,3-PENTADIENYL)-2-METHYL-4-OXO-, .DELTA.-LACTONE; 4H,3-b]pyran-4,5-dione, 2,3-dihydro-3-hydroxy-2-methyl-7-(1-propenyl)-, [2S-[2.alpha.,3.beta.,7(E)]]-; 4H,5H-PYRANO(4,3-B)PYRAN-4,5-DIONE, 2,3-DIHYDRO-3-HYDROXY-2-METHYL-7-((1E)-1-PROPEN-1-YL)-, (2S,3S)-
|
|
| CAS | 10088-95-6 | |
| PubChem CID | 5381458 | |
| ChEMBL ID | CHEMBL1994984 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 236.22 | ALogp: | 1.1 |
| HBD: | 1 | HBA: | 5 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 72.8 | Aromatic Rings: | 2 |
| Heavy Atoms: | 17 | QED Weighted: | 0.798 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.789 | MDCK Permeability: | 0.00001400 |
| Pgp-inhibitor: | 0.191 | Pgp-substrate: | 0.084 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.848 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.129 | Plasma Protein Binding (PPB): | 76.05% |
| Volume Distribution (VD): | 0.689 | Fu: | 23.05% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.883 | CYP1A2-substrate: | 0.718 |
| CYP2C19-inhibitor: | 0.112 | CYP2C19-substrate: | 0.59 |
| CYP2C9-inhibitor: | 0.054 | CYP2C9-substrate: | 0.906 |
| CYP2D6-inhibitor: | 0.069 | CYP2D6-substrate: | 0.857 |
| CYP3A4-inhibitor: | 0.028 | CYP3A4-substrate: | 0.196 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.482 | Half-life (T1/2): | 0.292 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.006 | Human Hepatotoxicity (H-HT): | 0.846 |
| Drug-inuced Liver Injury (DILI): | 0.969 | AMES Toxicity: | 0.026 |
| Rat Oral Acute Toxicity: | 0.108 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.163 | Carcinogencity: | 0.428 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.037 |
| Respiratory Toxicity: | 0.413 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004981 | 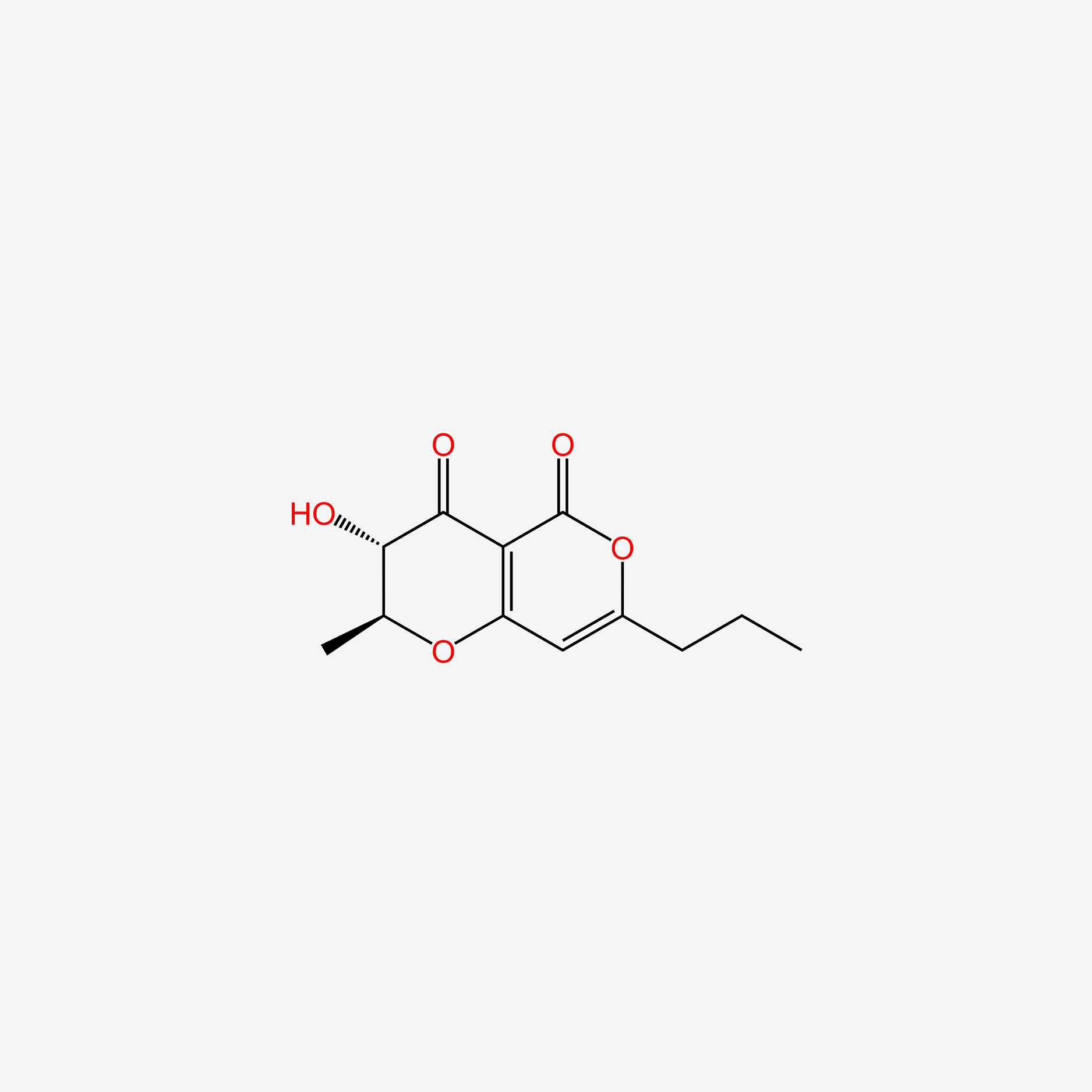 |
0.614 | D03KXY | 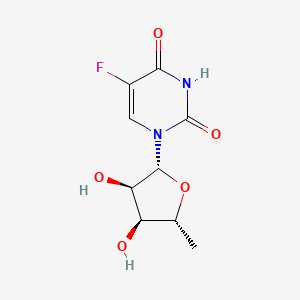 |
0.213 | ||
| ENC004982 | 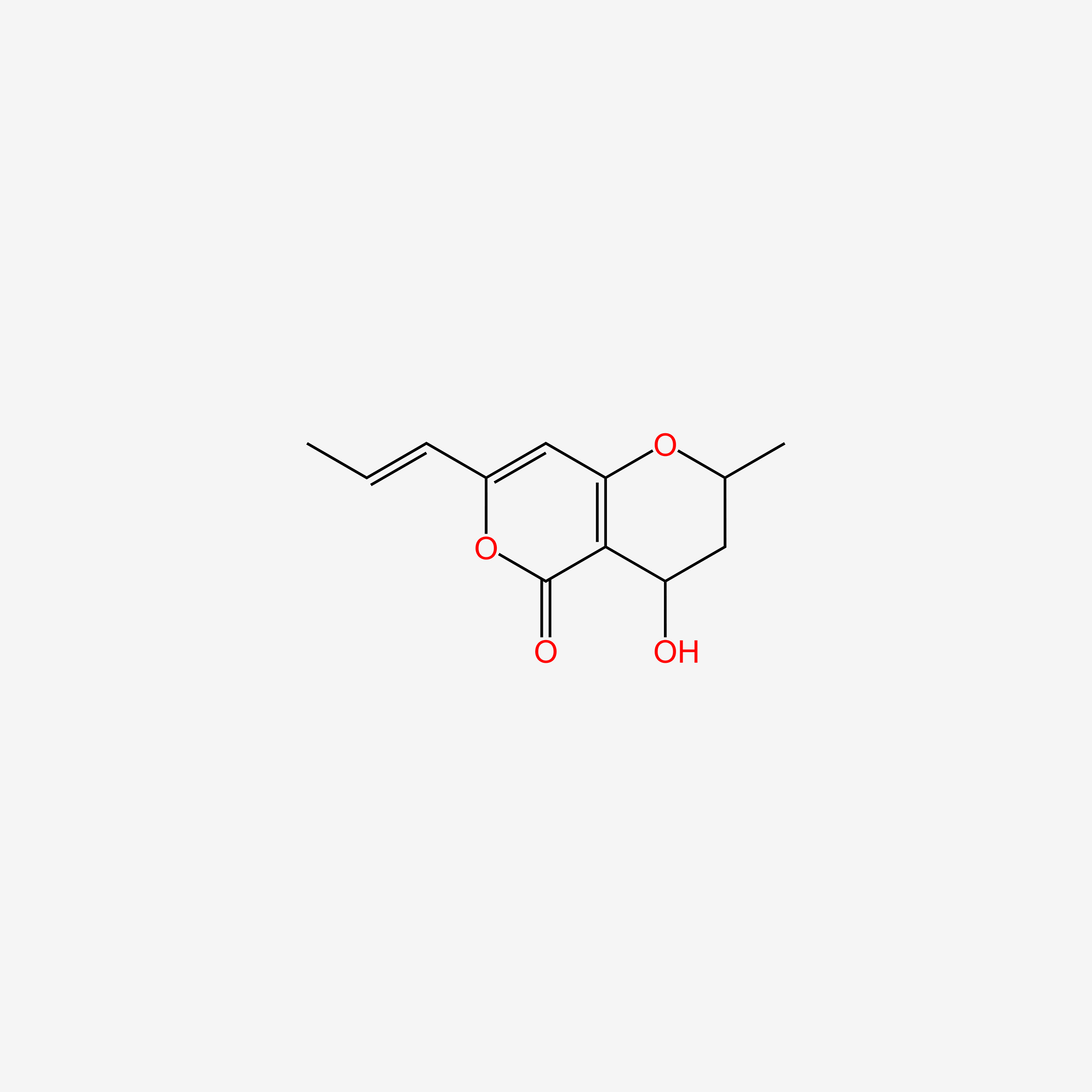 |
0.552 | D0K7LU | 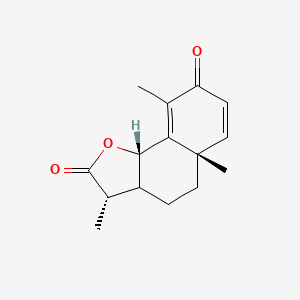 |
0.203 | ||
| ENC002796 | 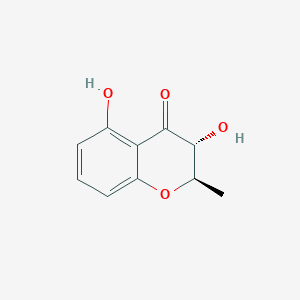 |
0.400 | D03TGJ | 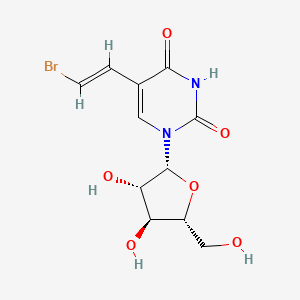 |
0.190 | ||
| ENC004404 | 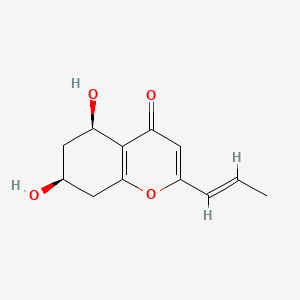 |
0.364 | D0AZ8C | 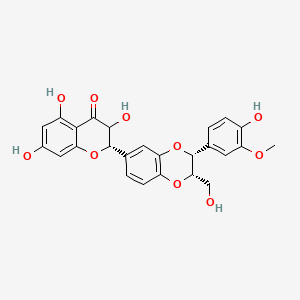 |
0.190 | ||
| ENC006074 | 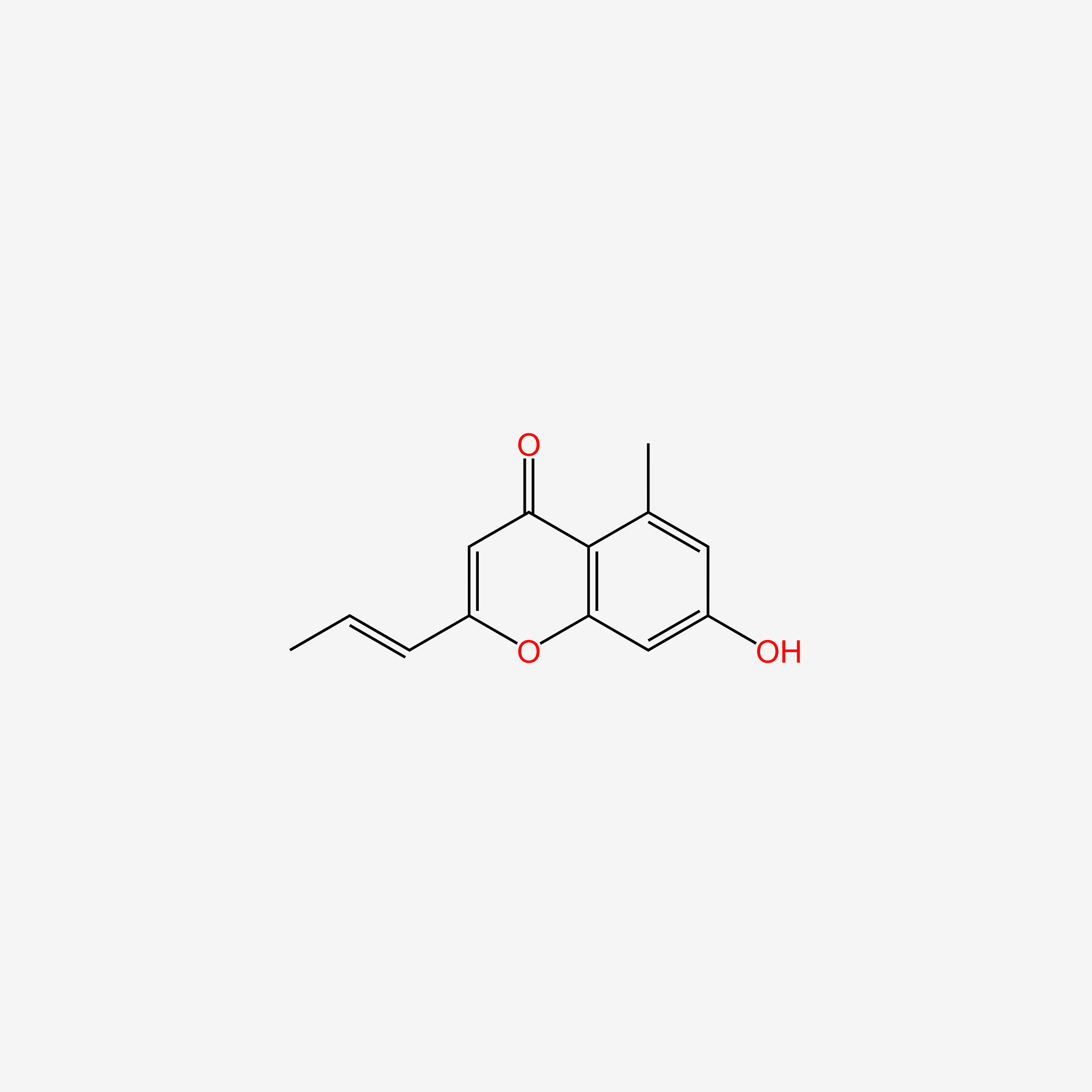 |
0.343 | D07MGA |  |
0.189 | ||
| ENC004363 | 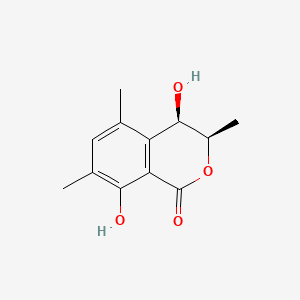 |
0.333 | D0YX4S | 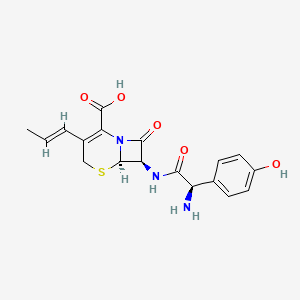 |
0.188 | ||
| ENC004991 | 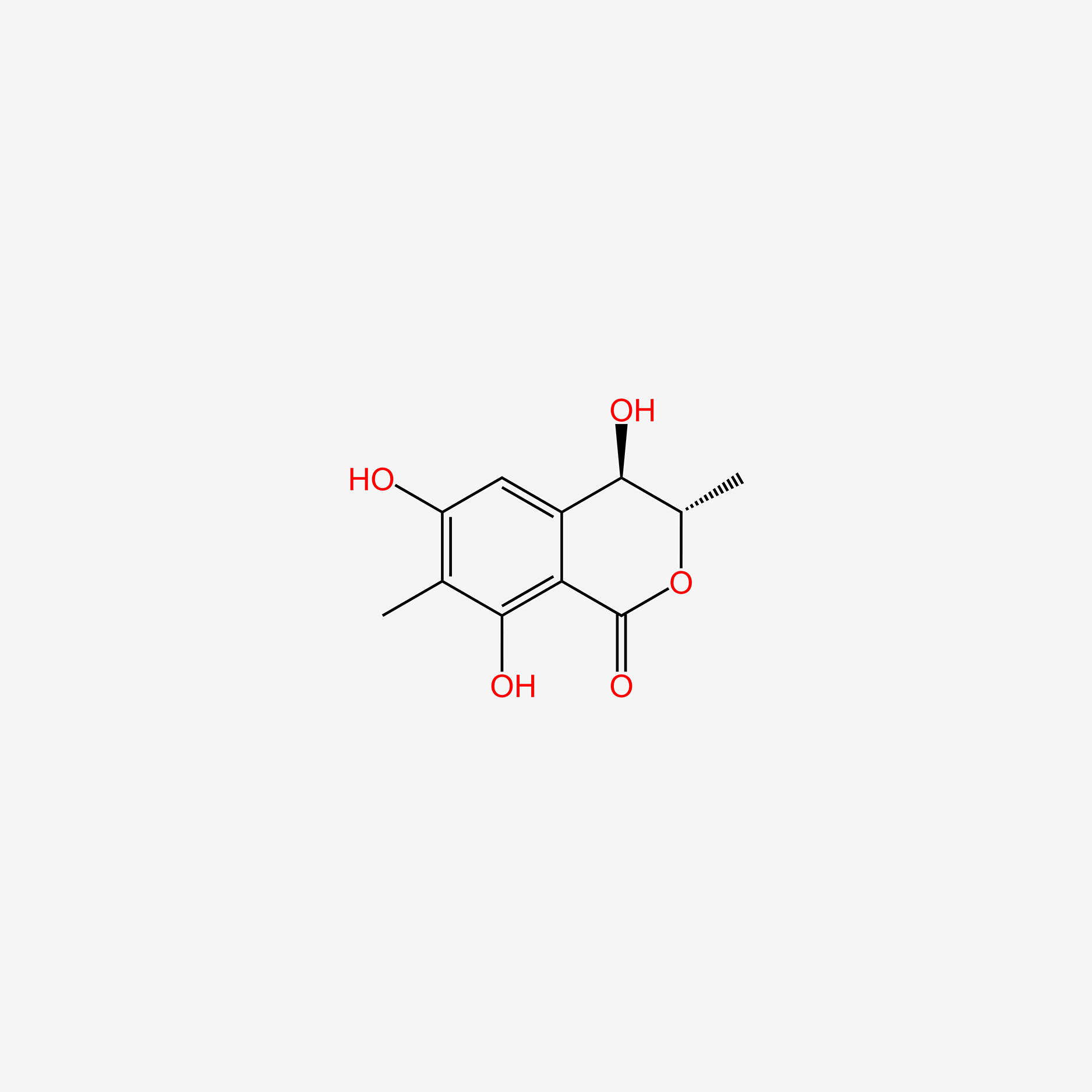 |
0.333 | D0G6AB | 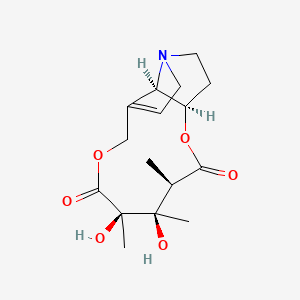 |
0.187 | ||
| ENC002384 | 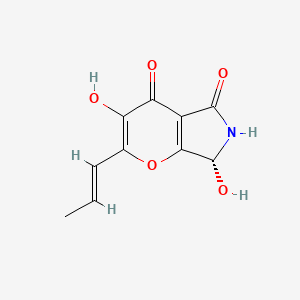 |
0.328 | D0R2KF | 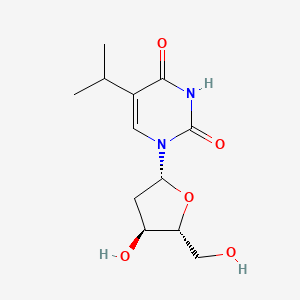 |
0.183 | ||
| ENC002669 | 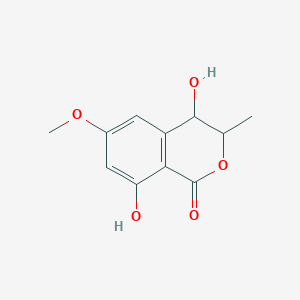 |
0.328 | D0C1SF | 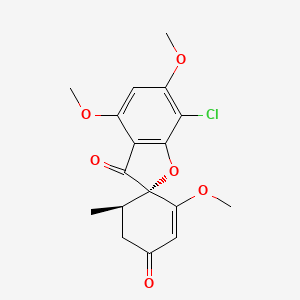 |
0.181 | ||
| ENC004881 | 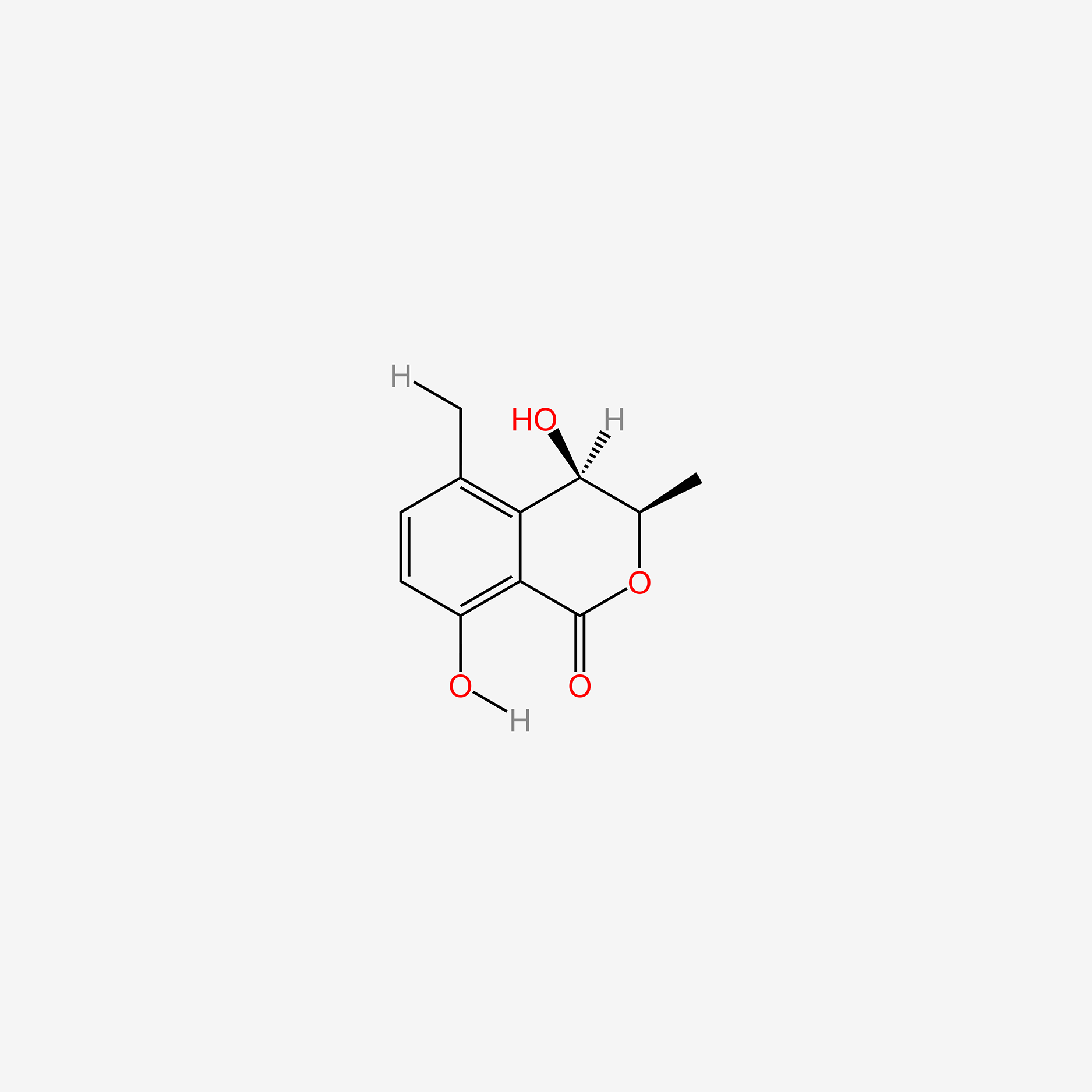 |
0.323 | D0CL9S | 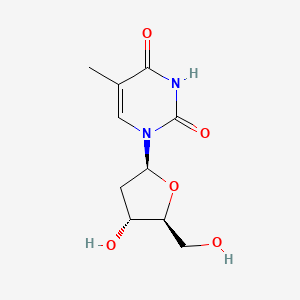 |
0.179 | ||