NPs Basic Information
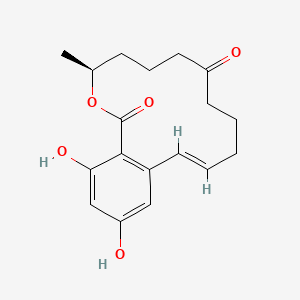
|
Name |
Zearalenone
|
| Molecular Formula | C18H22O5 | |
| IUPAC Name* |
(4S,12E)-16,18-dihydroxy-4-methyl-3-oxabicyclo[12.4.0]octadeca-1(14),12,15,17-tetraene-2,8-dione
|
|
| SMILES |
C[C@H]1CCCC(=O)CCC/C=C/C2=C(C(=CC(=C2)O)O)C(=O)O1
|
|
| InChI |
InChI=1S/C18H22O5/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(20)11-16(21)17(13)18(22)23-12/h3,7,10-12,20-21H,2,4-6,8-9H2,1H3/b7-3+/t12-/m0/s1
|
|
| InChIKey |
MBMQEIFVQACCCH-QBODLPLBSA-N
|
|
| Synonyms |
ZEARALENONE; 17924-92-4; trans-Zearalenone; (-)-Zearalenone; Mycotoxin F2; (S)-Zearalenone; Zenone; (S)-(-)-Zearalenone; (10S)-Zearalenone; (3S,11E)-14,16-dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione; CHEBI:10106; Compound F-2; MFCD00133085; 6-(10-Hydroxy-6-oxo-trans-1-undecenyl)-beta-resorcylic acid lactone; MLS000097901; CHEMBL454173; 1H-2-Benzoxacyclotetradecin-1,7(8H)-dione, 3,4,5,6,9,10-hexahydro-14,16-dihydroxy-3-methyl-, (3S,11E)-; F-2 toxin; 5W827M159J; (S-(E))-3,4,5,6,9,10-Hexahydro-14,16-dihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1,7(8H)-dione; SMR000058177; DSSTox_CID_1460; DSSTox_RID_76167; DSSTox_GSID_21460; Mycotoxin F-2; ZEA; CCRIS 623; Zearalenone 100 microg/mL in Acetonitrile; CAS-17924-92-4; HSDB 4208; NCI-C50226; 1H-2-Benzoxacyclotetradecin-1,7(8H)-dione, 3,4,5,6,9,10-hexahydro-14,16-dihydroxy-3-methyl-, (S)-(-)-; EINECS 241-864-0; BRN 1350216; UNII-5W827M159J; (2E,11S)-15,17-dihydroxy-11-methyl-12-oxabicyclo[12.4.0]octadeca-1(14),2,15,17-tetraene-7,13-dione; (S,E)-14,16-Dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-benzo[c][1]oxacyclotetradecine-1,7(8H)-dione; benzoxacyclotetradecin-1,7(8H)-dione; ZEARALENONE [MI]; Opera_ID_1608; ZEARALENONE [HSDB]; COMPD F-2; Epitope ID:146094; Benzoxacyclotetradec-11-en-1-one, 14,16-dihydroxy-3-methyl-7-oxo-, trans-; Resorcylic acid, 6-(10-hydroxy-6-oxo-1-undecenyl)-, mu-lactone, trans-; SCHEMBL33754; SCHEMBL33755; BSPBio_003581; Zearalenone, fungal mycotoxin; MLS000028817; MLS000888273; MLS001174881; BIDD:ER0058; SPECTRUM1505290; Zearalenone, reference material; 6-(10-Hydroxy-6-oxo-trans-1-undecenyl)-.beta.-resorcylic acid lactone; DTXSID0021460; REGID_for_CID_657988; REGID_for_CID_5281576; ALBB-030793; BCP06062; EX-A3324; ZINC3881412; Tox21_201153; Tox21_303510; BBL010576; BDBM50247676; CCG-40041; HB2517; LMPK04000016; s5676; STK033813; AKOS001577898; SDCCGMLS-0066858.P001; NCGC00038520-03; NCGC00090809-01; NCGC00090809-02; NCGC00257246-01; NCGC00258705-01; (2E,11S)-15,17-dihydroxy-11-methyl-12-oxabicyclo[12.4.0]octadeca-1(18),2,14,16-tetraene-7,13-dione; (3S,11E)-3,4,5,6,9,10-Hexahydro-14,16-dihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1,7(8H)-dione; (S-(Z))-3,4,5,6,9,10-Hexahydro-14,16-dihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1,7(8H)-dione; 1H-2-Benzoxacyclotetradecin-1,7(8H)-dione, 3,4,5,6,9,10-hexahydro-14,16-dihydroxy-3-methyl-, (S-(Z))-; AC-35060; VS-02573; HY-103447; CS-0027914; Z0047; C09981; 924Z924; Q169326; J-011439; BRD-K42017082-001-02-6; 6-(10-HYDROXY-6-OXO-TRANS-1-UNDECENYL) BETA RESORCYCLIC ACID-MU-LACTONE; (3S,11E)-14,16-dihydroxy-3-methyl-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecine-1,7-dione; (3S,11E)-3,4,5,6,9,10-Hexahydro-14, 16-dihydroxy-3-methyl-1H-2-benzoxacyclotetradecin- 1,7(8H)-dione; (4S,12E)-16,18-dihydroxy-4-methyl-3-oxabicyclo[12.4.0]octadeca-1(14),12,15,17-tetraene-2,8-dione; (S,E)-14,16-Dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione; 1H-2-Benzoxacyclotetradecin-1,7(8H)-dione, 3,4,5,6,9,10-hexahydro-14,16-dihydroxy-3-methyl-, (3S)-; 2beta-Methyl-15,17-dihydroxy-12,13-butano-1-oxacyclotetradecane-10,15(13),16,18(12)-tetraene-6,14-dione; 3,4,5,6,9,10-Hexahydro-14,16-dihydroxy-3-methyl-1H-2- benzoxacyclotetradecin-1,7(8H)-dione
|
|
| CAS | 17924-92-4 | |
| PubChem CID | 5281576 | |
| ChEMBL ID | CHEMBL454173 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 318.4 | ALogp: | 3.6 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.8 | Aromatic Rings: | 2 |
| Heavy Atoms: | 23 | QED Weighted: | 0.697 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.712 | MDCK Permeability: | 0.00002590 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.01 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.018 |
| 30% Bioavailability (F30%): | 0.144 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.101 | Plasma Protein Binding (PPB): | 94.55% |
| Volume Distribution (VD): | 0.812 | Fu: | 3.85% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.866 | CYP1A2-substrate: | 0.147 |
| CYP2C19-inhibitor: | 0.277 | CYP2C19-substrate: | 0.055 |
| CYP2C9-inhibitor: | 0.365 | CYP2C9-substrate: | 0.95 |
| CYP2D6-inhibitor: | 0.918 | CYP2D6-substrate: | 0.65 |
| CYP3A4-inhibitor: | 0.652 | CYP3A4-substrate: | 0.116 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 15.651 | Half-life (T1/2): | 0.927 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.018 | Human Hepatotoxicity (H-HT): | 0.189 |
| Drug-inuced Liver Injury (DILI): | 0.205 | AMES Toxicity: | 0.046 |
| Rat Oral Acute Toxicity: | 0.017 | Maximum Recommended Daily Dose: | 0.86 |
| Skin Sensitization: | 0.535 | Carcinogencity: | 0.088 |
| Eye Corrosion: | 0.017 | Eye Irritation: | 0.442 |
| Respiratory Toxicity: | 0.321 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC003872 | 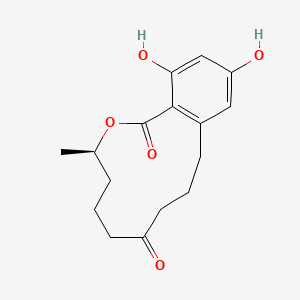 |
0.718 | D07MGA |  |
0.302 | ||
| ENC005002 | 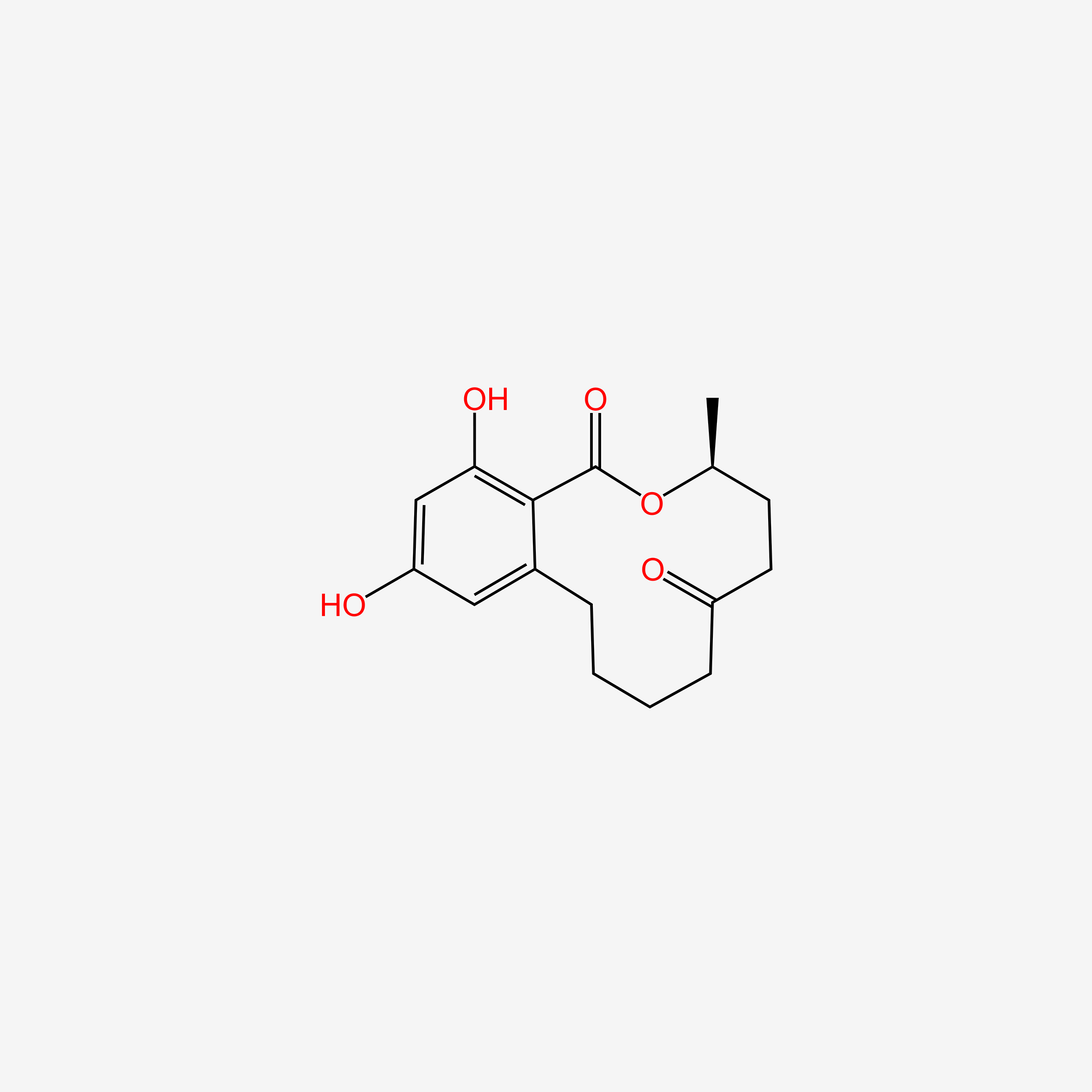 |
0.649 | D00ZFP | 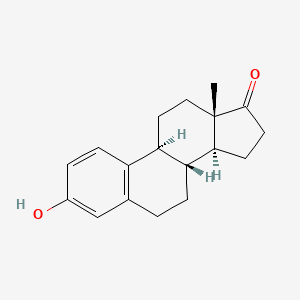 |
0.247 | ||
| ENC002425 | 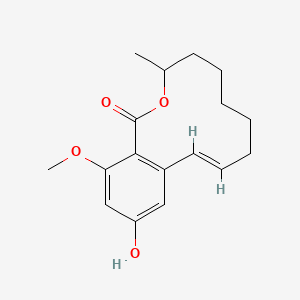 |
0.597 | D07GRH | 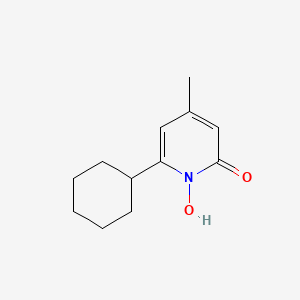 |
0.233 | ||
| ENC005419 | 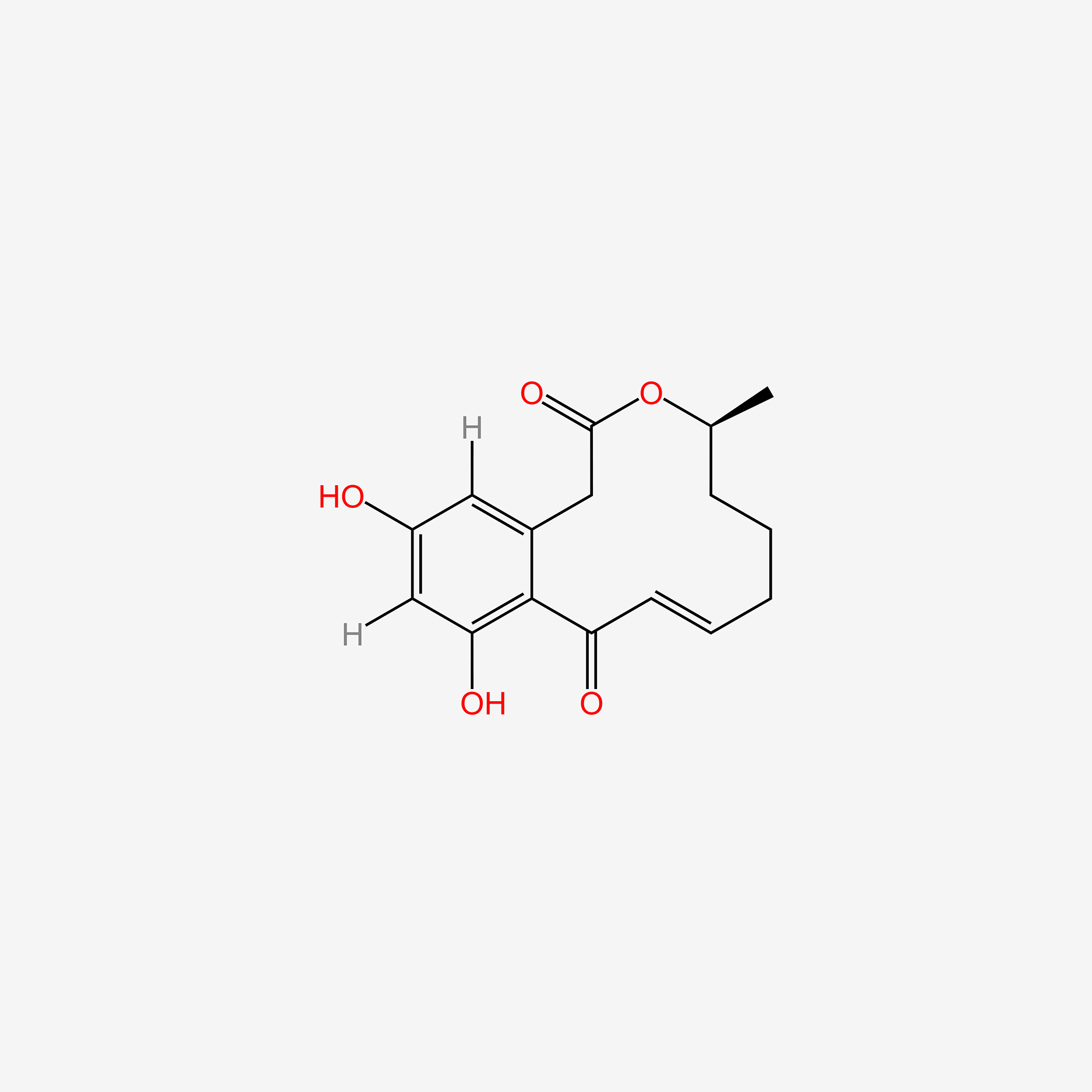 |
0.584 | D04AIT |  |
0.232 | ||
| ENC005417 | 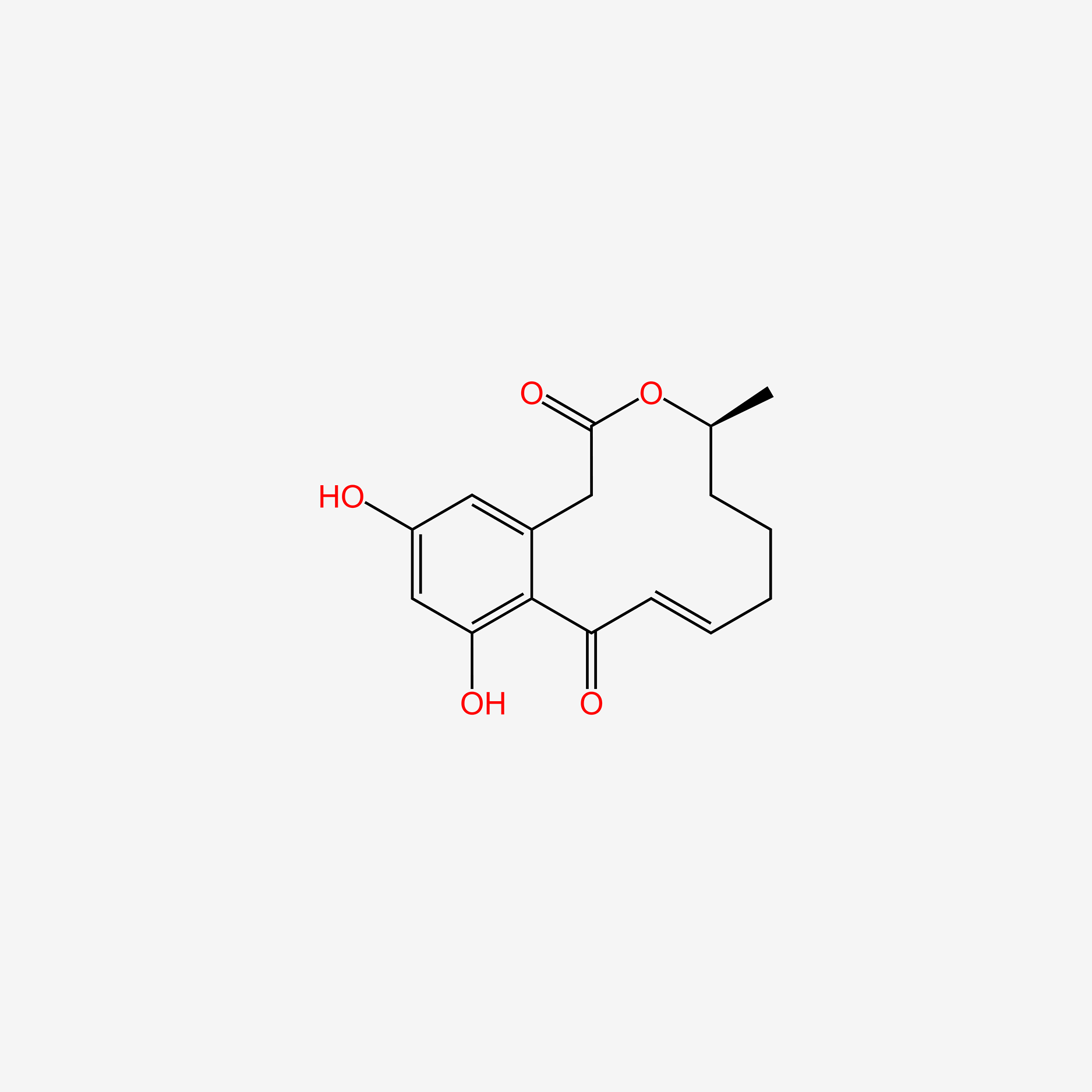 |
0.584 | D0K8KX |  |
0.228 | ||
| ENC001849 | 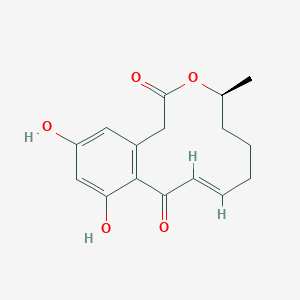 |
0.584 | D04JHN |  |
0.228 | ||
| ENC003871 | 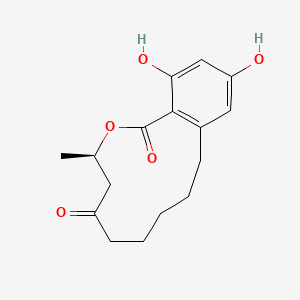 |
0.584 | D0P6VV | 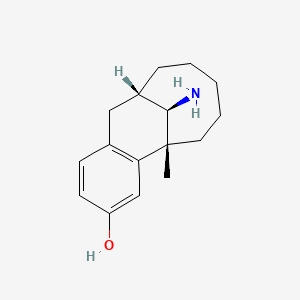 |
0.223 | ||
| ENC005643 | 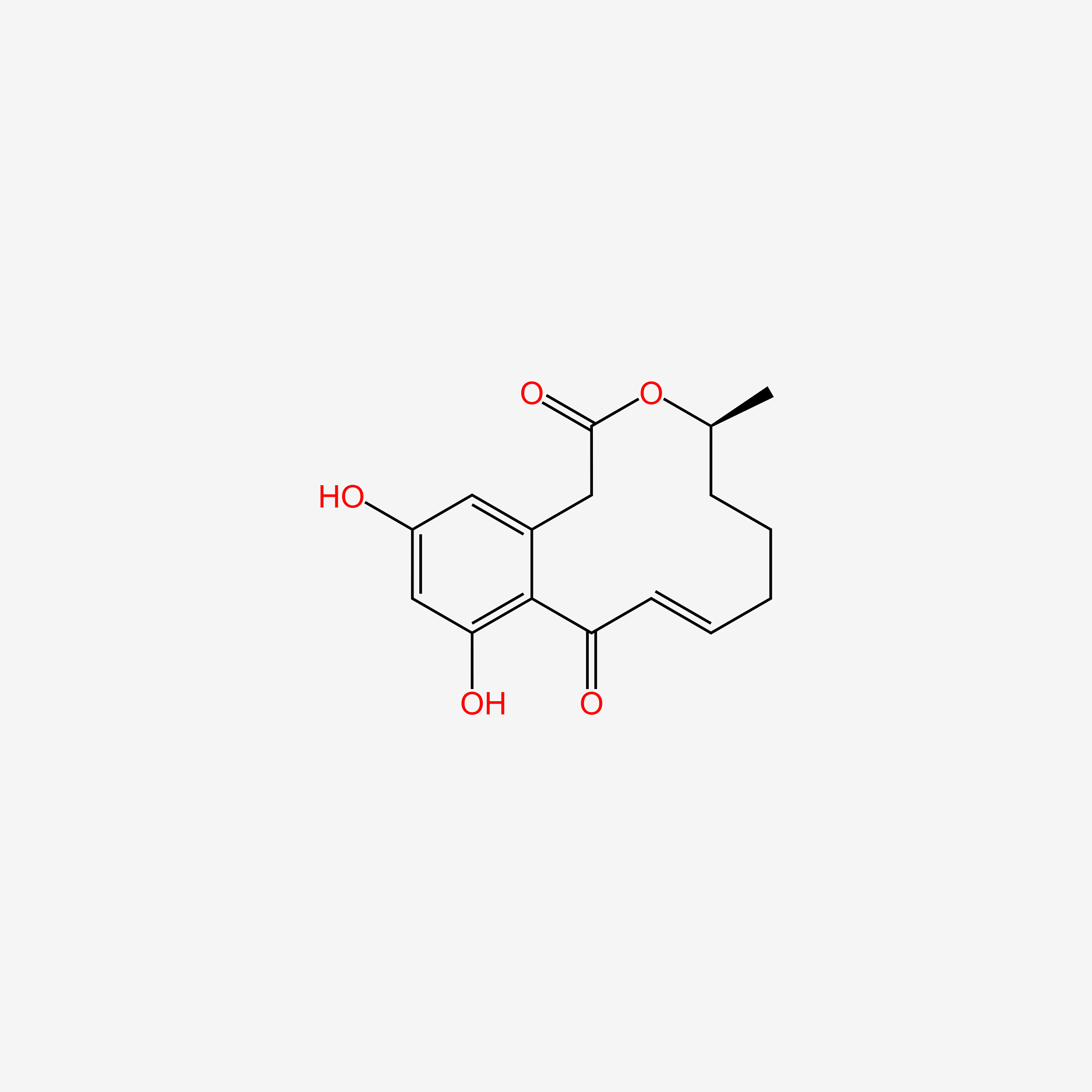 |
0.584 | D0C7JF |  |
0.223 | ||
| ENC002287 |  |
0.584 | D02NSF |  |
0.223 | ||
| ENC002286 |  |
0.584 | D0H6QU | 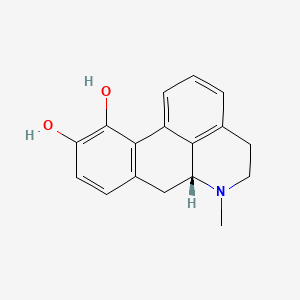 |
0.222 | ||