| Synonyms |
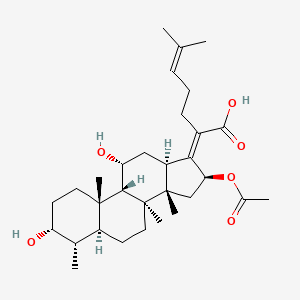
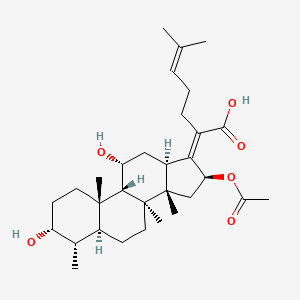
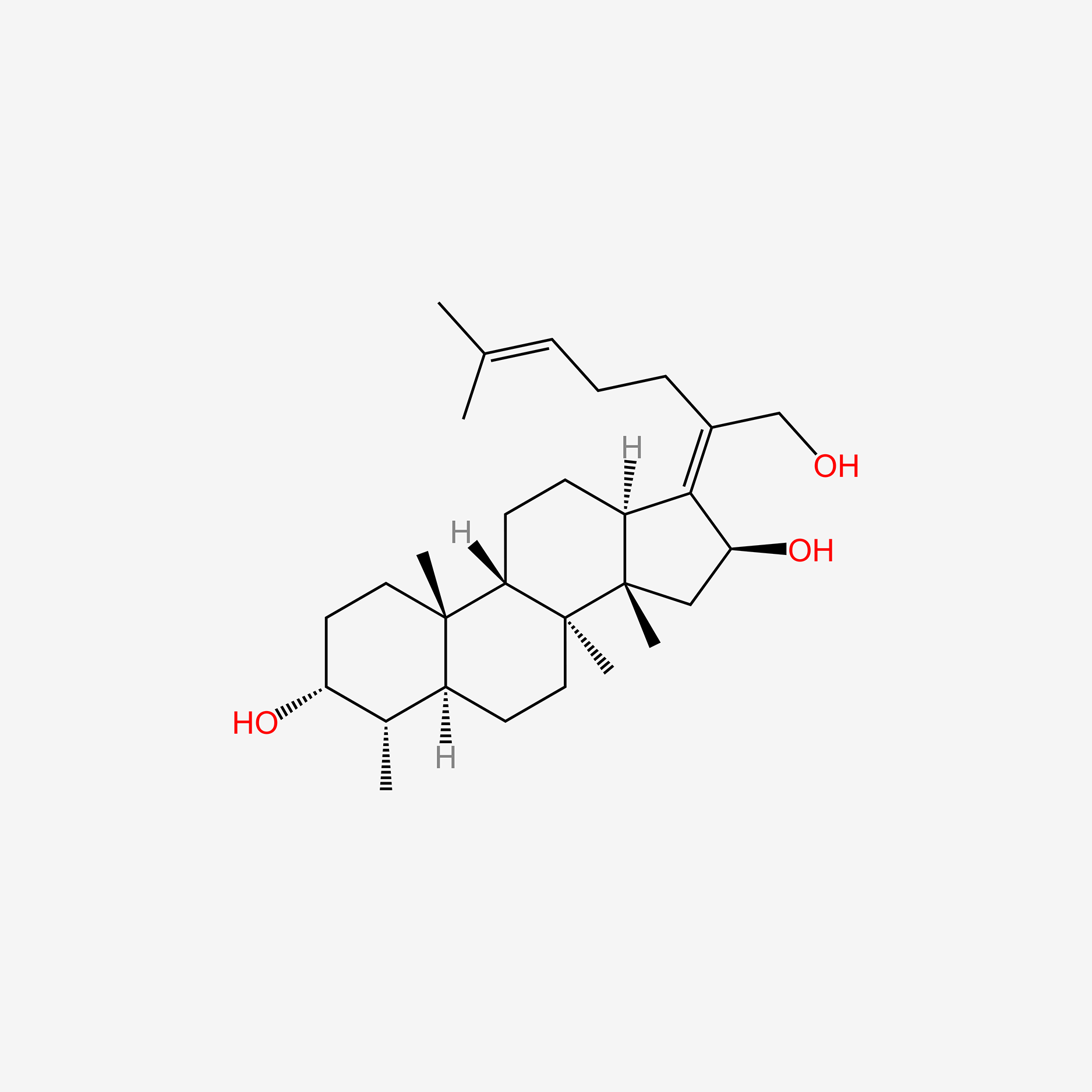
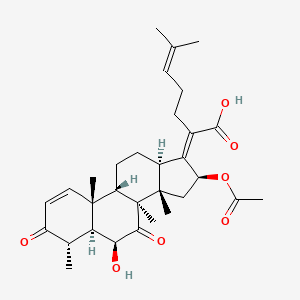
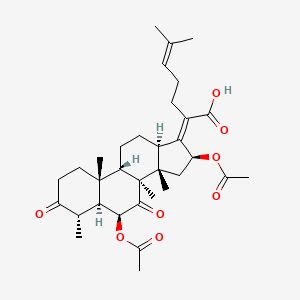
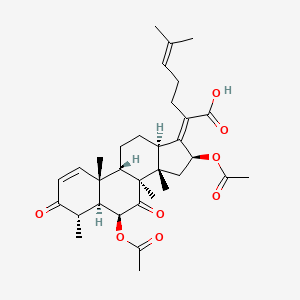
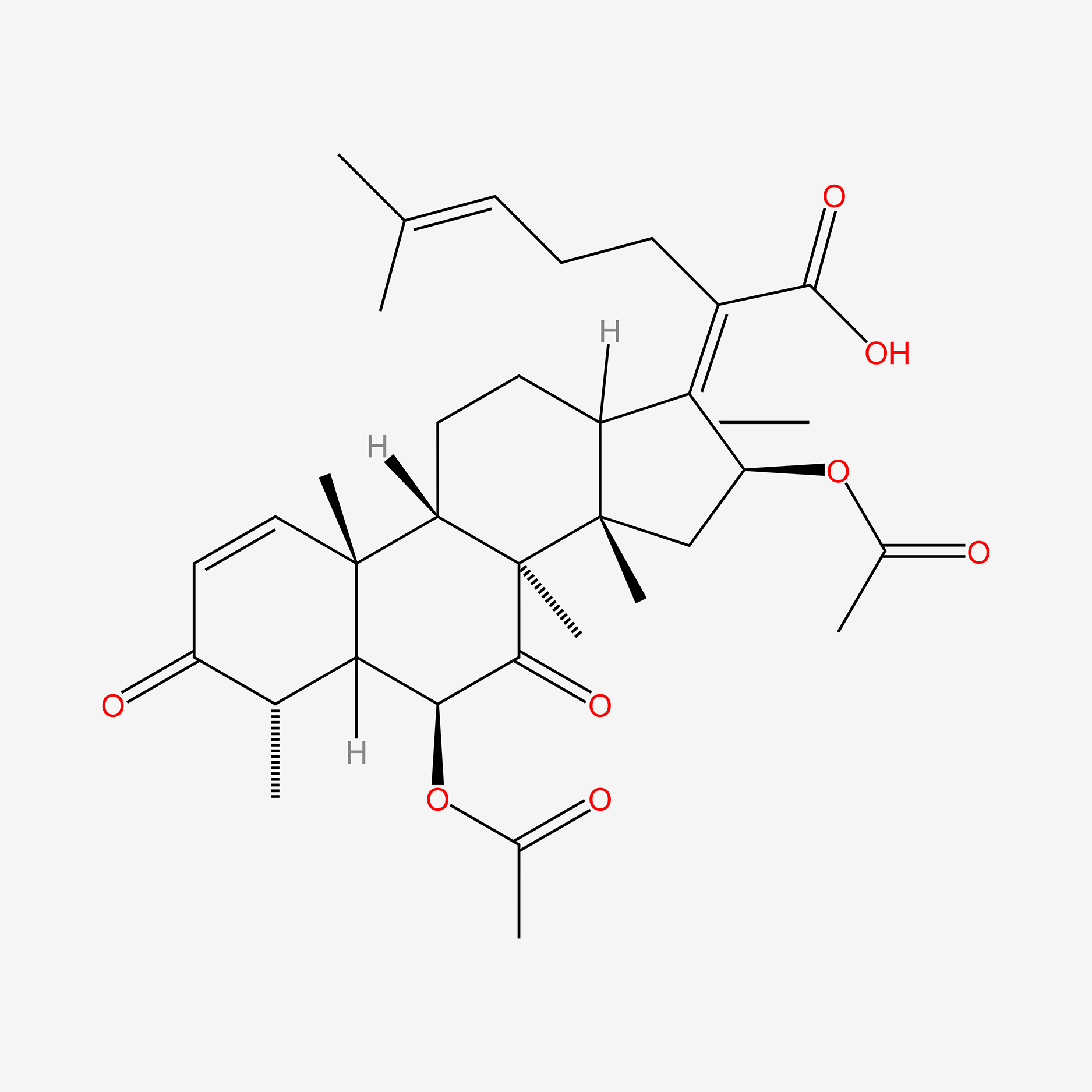
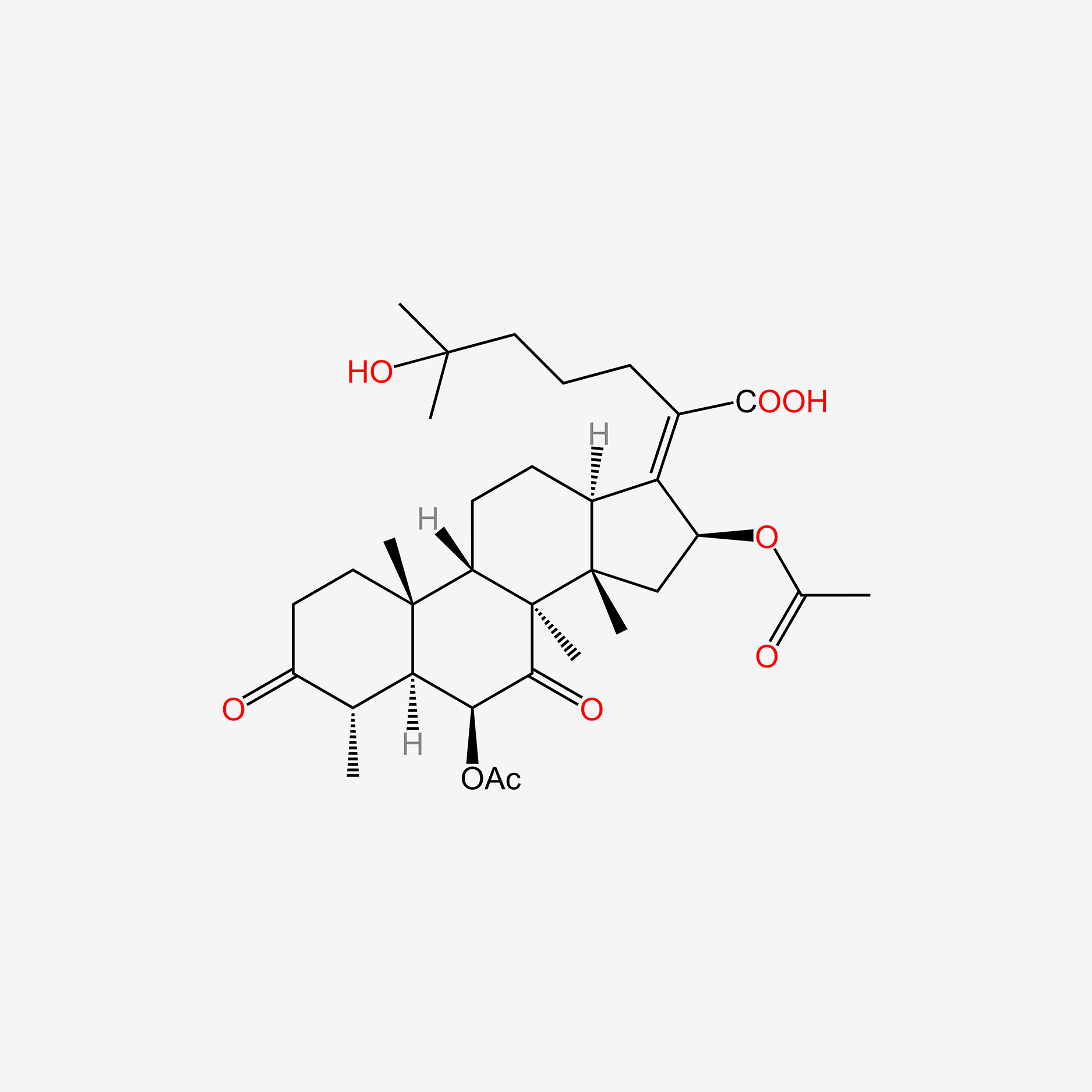
fusidic acid; 6990-06-3; Fusidine; Ramycin; Fucithalmic; Fusidate; Fucidic acid; Fucidin acid; FUCIDIN; Flucidin; Fucidate; Taksta; Sodium fusidate; SQ 16,603; (-)-Fusidic acid; (2Z)-2-[(3R,4S,5S,8S,9S,10S,11R,13R,14S,16S)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoic acid; Anhydrous Fusidic Acid; CEM-102; NSC-56192; SQ 16603; MLS001332649; CHEBI:29013; 59XE10C19C; SMR000857101; SQ-16603; (2E)-2-[(3R,4S,5S,8S,10S,11R,13R,14S,16S)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoic acid; Acide fusidique; Acido fusidico; Fusidinic Acid; Acidum fusidicum; MFCD00865135; (-)-16beta-acetoxy-3alpha,11alpha-dihydroxyfusida-17(20)Z,24-diene-21-oic acid; (2Z)-2-[(3alpha,4alpha,5alpha,8alpha,9beta,11alpha,13alpha,14beta,16beta,17Z)-16-(acetyloxy)-3,11-dihydroxy-4,8,10,14-tetramethylgonan-17-ylidene]-6-methylhept-5-enoic acid; (2Z)-2-[(3R,4S,5S,8S,9S,10S,11R,13R,14S,16S)-16-acetoxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-ylidene]-6-methyl-hept-5-enoic acid; (3alpha,4alpha,8alpha,9beta,11alpha,13alpha,14beta,16beta,17Z)-16-(Acetyloxy)-3,11-dihydroxy-29-nordammara-17(20),24-dien-21-oic acid; (Z)-2-((3R,4S,5S,8S,9S,10S,11R,13R,14S,16S)-16-acetoxy-3,11-dihydroxy-4,8,10,14-tetramethyldodecahydro-1H-cyclopenta[a]phenanthren-17(2H,10H,14H)-ylidene)-6-methylhept-5-enoic acid; 3alpha,11alpha,16beta-Trihydroxy-29-nor-8alpha,9beta,13alpha,14beta-dammara-17(20),24-dien-21-oic acid 16-acetate; Fusidic acid (USAN/INN); fusidic-acid; Fusidate Acid; 1qca; FUSIDICACID; C.A.S. 62,602; Prestwick2_000390; FUSIDIC ACID [MI]; FUSIDIC ACID [INN]; FUSIDIC ACID [USAN]; SCHEMBL25646; MLS001332650; MLS002207094; UNII-59XE10C19C; Acide fusidique [INN-French]; Acido fusidico [INN-Spanish]; Acidum fusidicum [INN-Latin]; FUSIDIC ACID [WHO-DD]; CEM102; CHEMBL374975; DTXSID0023086; Fusidic acid [USAN:INN:BAN]; BDBM58924; cid_3000226; GTPL10815; HMS2235B11; FUSIDIC ACID [EP IMPURITY]; ACT03304; EX-A3797; FUSIDIC ACID [EP MONOGRAPH]; HY-B1350; NSC56192; ZINC8143796; EINECS 230-256-0; Fusidic acid for peak identification; NSC 56192; s3971; 16-(Acetyloxy)-3,11-dihydroxy-29-nordammara-17(20),24-dien-21-oic acid; AKOS005146257; CCG-269829; DB02703; DS-3261; LMPR0106040001; NCGC00485232-01; (2Z)-2-[(17Z)-16beta-acetoxy-3alpha,11alpha-dihydroxy-4alpha,8alpha,10,14beta-tetramethyl-5alpha,9beta,13alpha-gonan-17-ylidene]-6-methylhept-5-enoic acid; 29-Nordammara-17(20),24-dien-21-oic acid, 16-(acetyloxy)-3,11-dihydroxy-, (3alpha,4alpha,8alpha,9beta,11alpha,13alpha,14beta,16beta,17Z)-; C.A.S. 62,602; Diethanolamine fusidate; CS-0013095; F1007; C06694; D04281; 990F063; EN300-22411576; Q259930; Q-201141; Fusidic acid, European Pharmacopoeia (EP) Reference Standard; Fusidic acid for peak identification, European Pharmacopoeia (EP) Reference Standard; (2Z)-2-[(3beta,4beta,5alpha,8alpha,9beta,11beta,13alpha,16beta,17Z)-16-(acetyloxy)-3,11-dihydroxy-4,8,10,14-tetramethylgonan-17-ylidene]-6-methylhept-5-enoic acid; (2Z)-2-[(3R,4S,5S,8S,9S,10S,11R,13R,14S,16S)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-ylidene]-6-methyl-5-heptenoic acid; (2Z)-2-[(3R,4S,5S,8S,9S,10S,11R,13R,14S,16S)-16-acetyloxy-4,8,10,14-tetramethyl-3,11-bis(oxidanyl)-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-ylidene]-6-methyl-hept-5-enoic acid; (3alpha,4alpha,8alpha,9beta,11alpha,13alpha,147beta,167beta,17Z)-16-(Acetyloxy)-3,11-dihydroxy-29-nordammara-17(20),24-dien-21-oic Acid; 2-[(1Z,2S,3aS,3bS,5aS,6S,7R,9aS,9bS,10R,11aR)-2-(acetyloxy)-7,10-dihydroxy-3a,3b,6,9a-tetramethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-1-ylidene]-6-methylhept-5-enoic acid; 29-Nor-8.alpha.,13.alpha.,14.beta.-dammara-17(20),24-dien-21-oic acid, 3.alpha.,11.alpha.,16.beta.-trihydroxy-, 16-acetate, (Z)-; 29-Nor-8alpha,9beta,13alpha,14beta-dammara-17(20),24-dien-21-oic acid, 3alpha,11alpha,16beta-trihydroxy-, 16-acetate, (Z)-; 29-Nordammara-17(20), 16-(acetyloxy)-3,11-dihydroxy-, (3.alpha.,4.alpha.,8.alpha.,9.beta.,11.alpha.,13.alpha.,14.beta.,16.beta.,17Z)-; 29-NORDAMMARA-17(20),24-DIEN-21-OIC ACID, 16-(ACETYLOXY)-3,11-DIHYDROXY-, (3.ALPHA.,4.ALPHA.,8.ALPHA.,9.BETA.,11.ALPHA.,13.ALPHA.,14.BETA.,16.BETA.,17Z); 29-Nordammara-17(20),24-dien-21-oic acid, 16-(acetyloxy)-3,11-dihydroxy-, (3a,4a,8a,9b,11a,13a,14b,16b,17Z)-; 3.alpha.,16.beta.-Trihydroxy-29-nor-8.alpha.,9.beta.,13.alpha.,14.beta.-dammara-17(20),24-dien-21-oic acid 16-acetate
|