NPs Basic Information
|
Name |
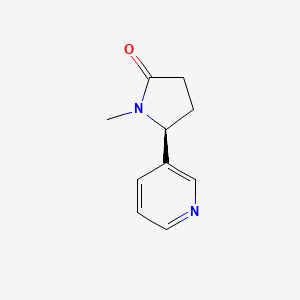
Cotinine
|
| Molecular Formula | C10H12N2O | |
| IUPAC Name* |
(5S)-1-methyl-5-pyridin-3-ylpyrrolidin-2-one
|
|
| SMILES |
CN1[C@@H](CCC1=O)C2=CN=CC=C2
|
|
| InChI |
InChI=1S/C10H12N2O/c1-12-9(4-5-10(12)13)8-3-2-6-11-7-8/h2-3,6-7,9H,4-5H2,1H3/t9-/m0/s1
|
|
| InChIKey |
UIKROCXWUNQSPJ-VIFPVBQESA-N
|
|
| Synonyms |
cotinine; (-)-Cotinine; 486-56-6; (S)-Cotinine; Cotinina; Cotininum; (S)-(-)-Cotinine; Cotinine [INN]; Cotinine (-); (S)-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one; (5S)-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one; (S)-1-Methyl-5-(3-pyridinyl)-2-pyrrolidinone; 2-Pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (5S)-; NIH 10498; (5S)-1-methyl-5-pyridin-3-ylpyrrolidin-2-one; CHEBI:68641; K5161X06LL; S-(-)-Cotinine; (-)-Cotinine 100 microg/mL in Acetonitrile; DSSTox_CID_27576; DSSTox_RID_82428; DSSTox_GSID_47576; (5~{S})-1-methyl-5-pyridin-3-yl-pyrrolidin-2-one; BRD4010; BRD-4010; (5S)-1-Methyl-5-(pyridin-3-yl)pyrrolidin-2-one ((-)-Cotinine); 2-Pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (S)-; SMR000449278; Cotininum [INN-Latin]; SR-01000075768; Cotinina [INN-Spanish]; UNII-K5161X06LL; CCRIS 7625; HSDB 7805; NCGC00093739-04; (5S)-1-methyl-5-(3-pyridinyl)-2-pyrrolidinone; CAS-486-56-6; Prestwick_134; EINECS 207-634-9; MFCD00077696; n-methyl-2-(3-pyridyl)-5-pyrrolidone; BRN 0083099; Spectrum_001984; COTININE [HSDB]; Nicotine EP Impurity C; COTININE [MI]; Prestwick0_000082; Prestwick1_000082; Prestwick2_000082; Prestwick3_000082; Spectrum3_000700; Spectrum4_001793; Spectrum5_000465; NICOTINE IMPURITY C; bmse000577; (-)-Cotinine, 98%; (S)-1-Methyl-5-(3-pyridyl)-2-pyrrolidinone; S(-)-1-Methyl-5-(3-pyridyl)-2-pyrrolidone; (5S)-1-methyl-5-(3-pyridyl)pyrrolidin-2-one; Lopac0_000285; SCHEMBL49060; BSPBio_000004; BSPBio_002459; KBioGR_002368; KBioSS_002550; 5-24-02-00504 (Beilstein Handbook Reference); MLS000758262; MLS001423950; DivK1c_000861; SPECTRUM1500208; SPBio_001943; (-)-Cotinine, >=98%; BPBio1_000006; CHEMBL578211; MEGxp0_001870; DTXSID1047576; ACon1_000202; KBio1_000861; KBio2_002541; KBio2_005109; KBio2_007677; KBio3_001679; NINDS_000861; US8609708, 91 Cotinine; HMS1568A06; HMS1920A14; HMS2051A15; HMS2091G22; HMS2095A06; HMS2232F15; HMS3260J12; Pharmakon1600-01500208; ZINC402766; (-)-Cotinine, analytical standard; HY-B1178; Tox21_111219; Tox21_300615; Tox21_500285; BBL102262; BDBM50370573; NIH-10498; NSC756704; s9339; STL556061; AKOS007930814; Tox21_111219_1; (-)-Cotinine 1.0 mg/ml in Methanol; CCG-100799; CS-4787; LP00285; NC00049; NSC-756704; SDCCGMLS-0066565.P001; SDCCGSBI-0050273.P004; IDI1_000861; NCGC00093739-08; NCGC00093739-13; NCGC00093739-20; NCGC00254396-01; NCGC00260970-01; AC-35718; AS-50387; NICOTINE IMPURITY C [EP IMPURITY]; SBI-0050273.P003; AM20061246; EU-0100285; C 5923; P10066; AB00053721_08; (5S)-1-methyl-5-pyridin-3-yl-pyrrolidin-2-one; A827581; NICOTINE RESINATE IMPURITY C [EP IMPURITY]; Q421177; SR-01000075768-1; SR-01000075768-5; SR-01000075768-6; (-)-1-METHYL-5-(3-PYRIDYL)-2-PYRROLIDINONE; BRD-K94144010-001-04-8; BRD-K94144010-001-05-5; BRD-K94144010-001-09-7; NICOTINE DITARTRATE DIHYDRATE IMPURITY C [EP IMPURITY]; U5H
|
|
| CAS | 486-56-6 | |
| PubChem CID | 854019 | |
| ChEMBL ID | CHEMBL578211 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 176.21 | ALogp: | -0.3 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 33.2 | Aromatic Rings: | 2 |
| Heavy Atoms: | 13 | QED Weighted: | 0.654 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.248 | MDCK Permeability: | 0.00003200 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.426 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.008 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.812 | Plasma Protein Binding (PPB): | 28.01% |
| Volume Distribution (VD): | 1.166 | Fu: | 70.97% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.03 | CYP1A2-substrate: | 0.777 |
| CYP2C19-inhibitor: | 0.086 | CYP2C19-substrate: | 0.798 |
| CYP2C9-inhibitor: | 0.032 | CYP2C9-substrate: | 0.601 |
| CYP2D6-inhibitor: | 0.006 | CYP2D6-substrate: | 0.439 |
| CYP3A4-inhibitor: | 0.132 | CYP3A4-substrate: | 0.571 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.662 | Half-life (T1/2): | 0.392 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.027 | Human Hepatotoxicity (H-HT): | 0.469 |
| Drug-inuced Liver Injury (DILI): | 0.896 | AMES Toxicity: | 0.017 |
| Rat Oral Acute Toxicity: | 0.282 | Maximum Recommended Daily Dose: | 0.944 |
| Skin Sensitization: | 0.812 | Carcinogencity: | 0.274 |
| Eye Corrosion: | 0.022 | Eye Irritation: | 0.676 |
| Respiratory Toxicity: | 0.094 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002540 | 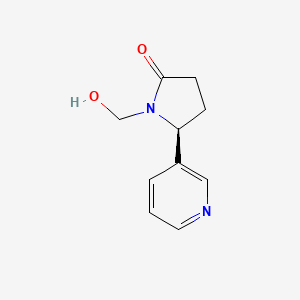 |
0.674 | D0TY5N | 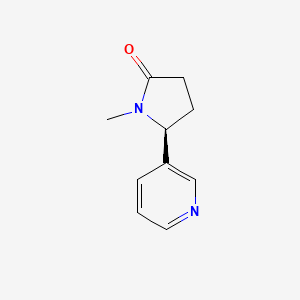 |
1.000 | ||
| ENC000048 | 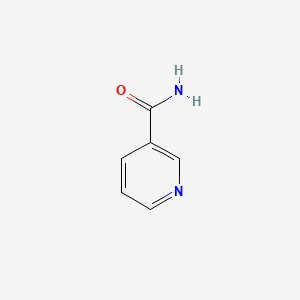 |
0.319 | D05QIM | 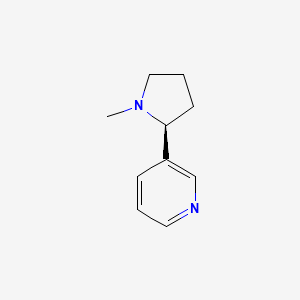 |
0.565 | ||
| ENC006142 | 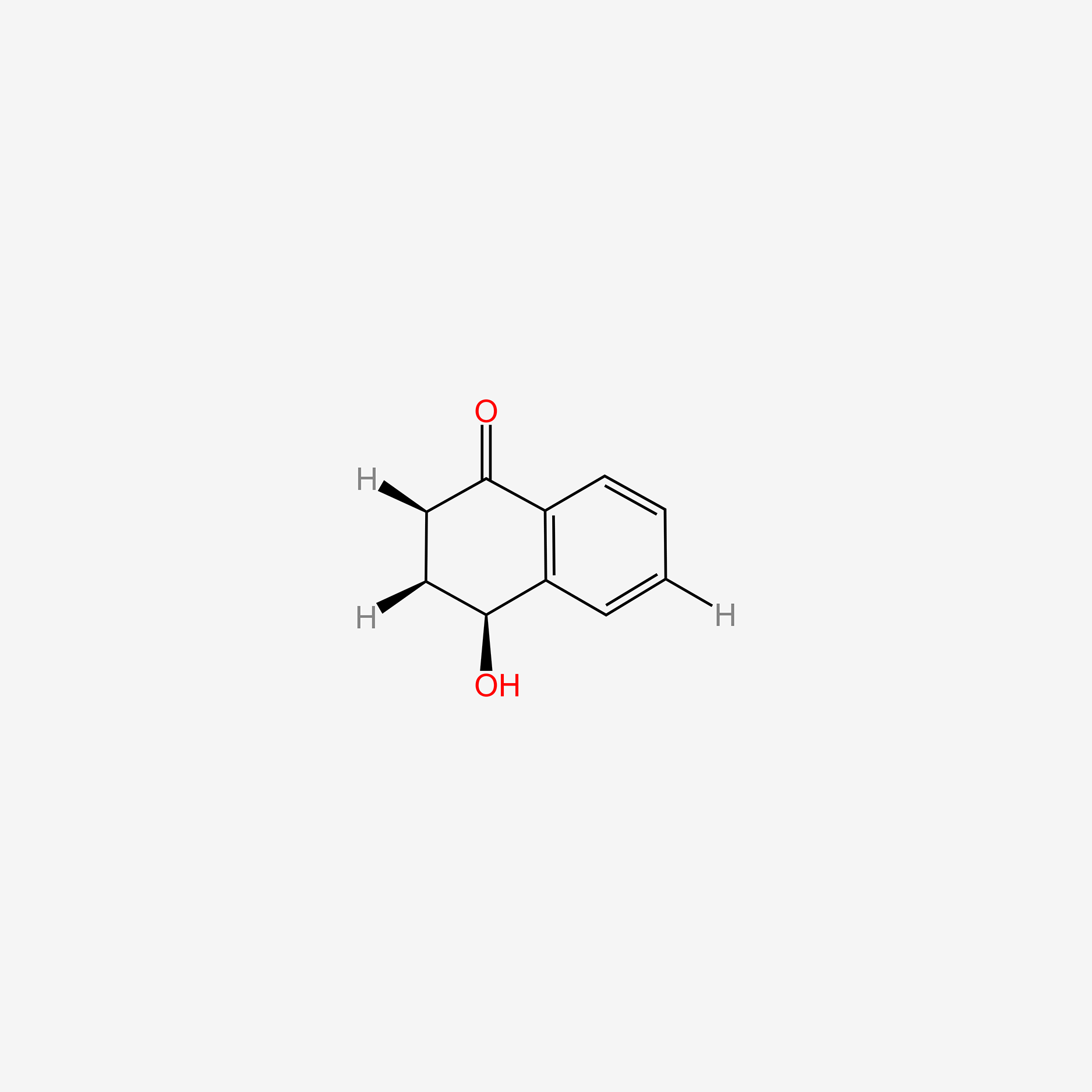 |
0.268 | D0T8LY | 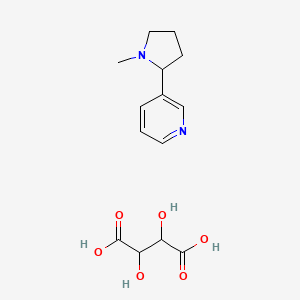 |
0.391 | ||
| ENC002450 | 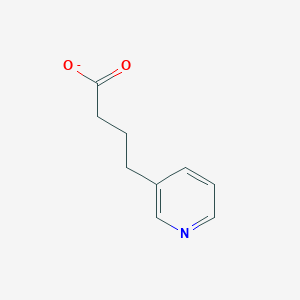 |
0.268 | D06NVJ | 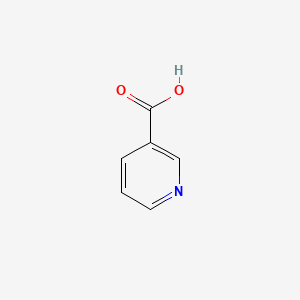 |
0.319 | ||
| ENC006050 | 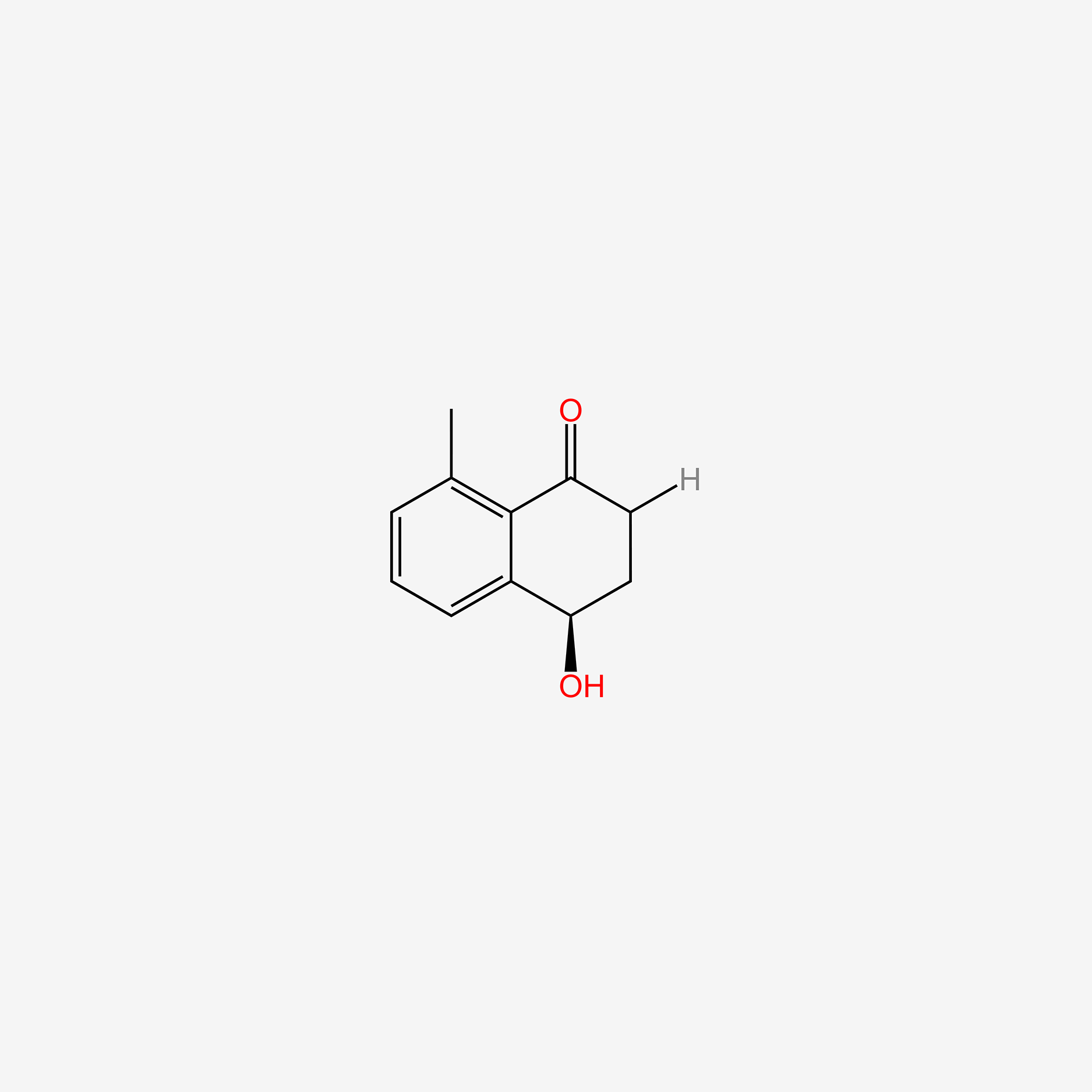 |
0.259 | D0S1OE | 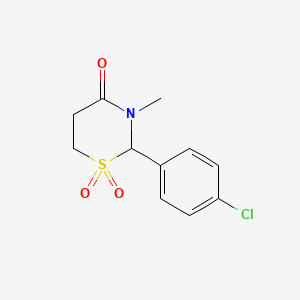 |
0.317 | ||
| ENC001516 | 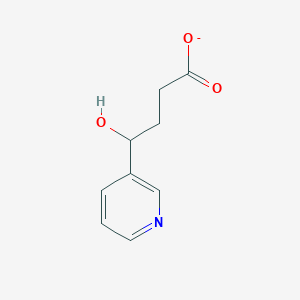 |
0.259 | D06BYV | 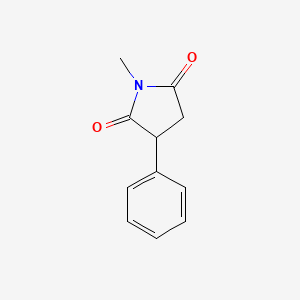 |
0.310 | ||
| ENC005321 | 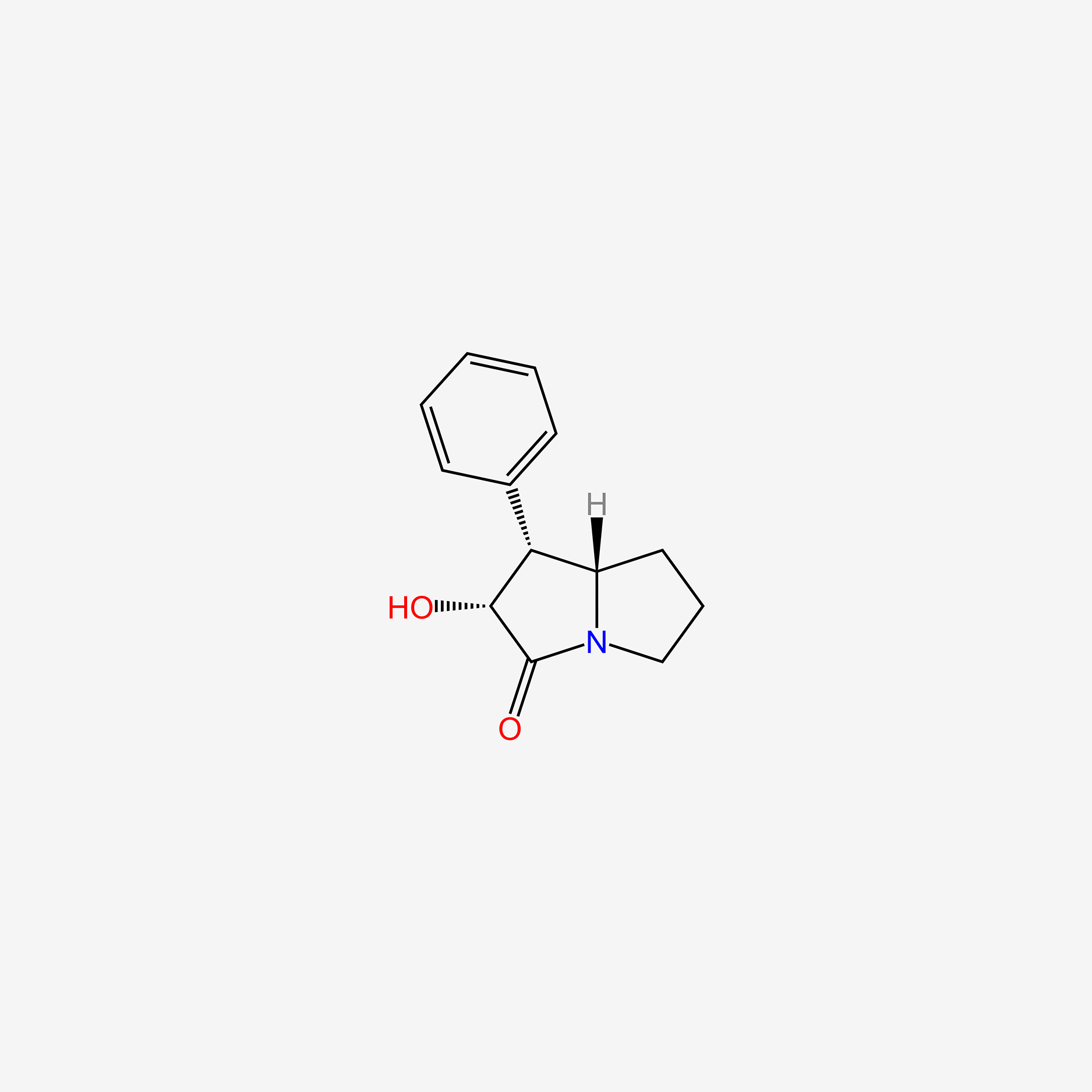 |
0.258 | D03AJU | 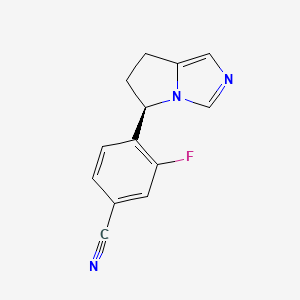 |
0.265 | ||
| ENC003135 | 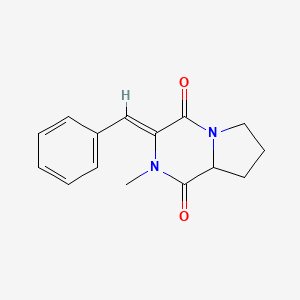 |
0.247 | D0ZX1P |  |
0.261 | ||
| ENC005721 |  |
0.246 | D06DLI |  |
0.254 | ||
| ENC002458 |  |
0.246 | D0O2EM | 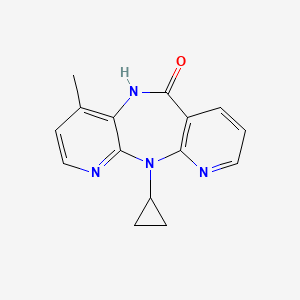 |
0.253 | ||