NPs Basic Information
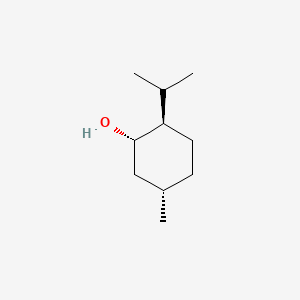
|
Name |
(+)-Menthol
|
| Molecular Formula | C10H20O | |
| IUPAC Name* |
(1S,2R,5S)-5-methyl-2-propan-2-ylcyclohexan-1-ol
|
|
| SMILES |
C[C@H]1CC[C@@H]([C@H](C1)O)C(C)C
|
|
| InChI |
InChI=1S/C10H20O/c1-7(2)9-5-4-8(3)6-10(9)11/h7-11H,4-6H2,1-3H3/t8-,9+,10-/m0/s1
|
|
| InChIKey |
NOOLISFMXDJSKH-AEJSXWLSSA-N
|
|
| Synonyms |
(+)-Menthol; 15356-60-2; d-Menthol; (1S,2R,5S)-(+)-Menthol; (1S,2R,5S)-Menthol; Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1S,2R,5S)-; (1S,2R,5S)-2-Isopropyl-5-methylcyclohexanol; Hexahydrothymol; Menthol, (+)-; (1S,2R,5S)-5-methyl-2-propan-2-ylcyclohexan-1-ol; (1S,2R,5S)-5-methyl-2-(propan-2-yl)cyclohexan-1-ol; Menthol, (1S,3S,4R)-(+)-; C6B1OE8P3W; rac-Menthol; CHEBI:76306; (+)-(1S,2R,5S)-menthol; (+)-(1S,3S,4R)-menthol; 89-78-1; p-Menthan-3-ol; MFCD00062983; (1S,2R,5S)-5-methyl-2-(1-methylethyl)cyclohexanol; (+)-p-Menthan-3-ol; Racemic menthol; 3-p-Menthanol; NSC 2603; Therapeutic mineral ice; Fisherman's friend lozenges; UNII-C6B1OE8P3W; Menthol, cis-1,3,trans-1,4-; Fancol menthol; Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.beta.,5.alpha.)-; (DL)-Menthol; EINECS 239-387-8; (.+/-.)-Menthol; (1S)-(+)-menthol; (+/-)-Menthol racemic; MENTHOL, D-; DSSTox_CID_9733; EC 239-387-8; DSSTox_RID_78817; DSSTox_GSID_29733; SCHEMBL521946; GTPL2471; CHEMBL2106989; DTXSID8029733; ZINC967511; (1s, 2r, 5s)-(+)-menthol; Tox21_201043; AKOS006281173; CS-W017993; DB11344; HY-W017277; NCGC00248905-01; NCGC00258596-01; AS-69562; (1S,2R,5S)-(+)-Menthol, 99%; CAS-15356-60-2; M0826; M3170; EN300-92162; A815982; (1S,2R,5S)-2-Isopropyl-5-methylcyclohexan-1-ol; (1S,2R,5S)-5-methyl-2-propan-2-yl-1-cyclohexanol; Q27084428; (1S,2R,5S)-5-methyl-2-propan-2-yl-cyclohexan-1-ol; F8889-8741; Z1198149783; 5.alpha.-Methyl-2.beta.-(1.alpha.-methylethyl)cyclohexanol; Cyclohexanol,5-methyl-2-(1-methylethyl)-,(1S,2R,5S)-; 2-Isopropyl-5-methylcyclohexanol, (1.alpha.,2.beta.,5.alpha.)-; 5-Methyl-2-(1-methylethyl)cyclohexanol, (1.alpha.,2.beta.,5.alpha.)-; Cyclohexanol, 2-isopropyl-5-methyl-, (1.alpha.,2.beta.,5.alpha.)-; Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1S-(1alpha,2beta,5alpha))-; (+)-Menthol, puriss. p.a., terpene standard for GC, >=99.0% (sum of enantiomers, GC)
|
|
| CAS | 89-78-1 | |
| PubChem CID | 165675 | |
| ChEMBL ID | CHEMBL2106989 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 156.26 | ALogp: | 3.0 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.618 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.429 | MDCK Permeability: | 0.00002290 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.823 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.839 |
| 30% Bioavailability (F30%): | 0.739 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.373 | Plasma Protein Binding (PPB): | 74.19% |
| Volume Distribution (VD): | 1.05 | Fu: | 20.56% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.395 | CYP1A2-substrate: | 0.595 |
| CYP2C19-inhibitor: | 0.028 | CYP2C19-substrate: | 0.899 |
| CYP2C9-inhibitor: | 0.092 | CYP2C9-substrate: | 0.677 |
| CYP2D6-inhibitor: | 0.006 | CYP2D6-substrate: | 0.171 |
| CYP3A4-inhibitor: | 0.022 | CYP3A4-substrate: | 0.31 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.777 | Half-life (T1/2): | 0.588 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.042 | Human Hepatotoxicity (H-HT): | 0.104 |
| Drug-inuced Liver Injury (DILI): | 0.225 | AMES Toxicity: | 0.027 |
| Rat Oral Acute Toxicity: | 0.081 | Maximum Recommended Daily Dose: | 0.028 |
| Skin Sensitization: | 0.811 | Carcinogencity: | 0.525 |
| Eye Corrosion: | 0.988 | Eye Irritation: | 0.985 |
| Respiratory Toxicity: | 0.873 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000578 | 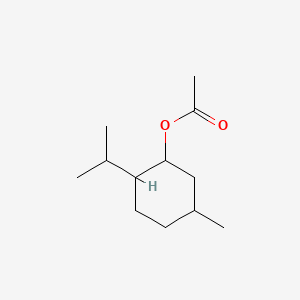 |
0.535 | D04CSZ | 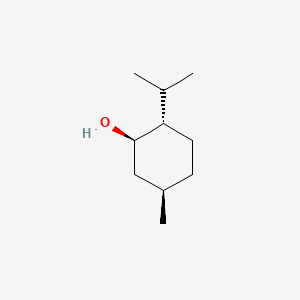 |
1.000 | ||
| ENC001908 | 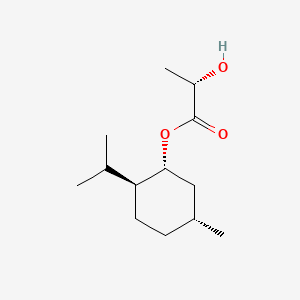 |
0.511 | D0V8HA | 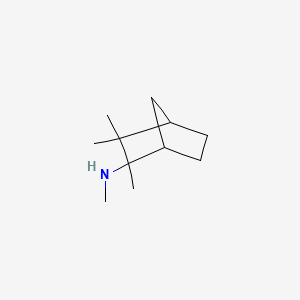 |
0.220 | ||
| ENC001888 | 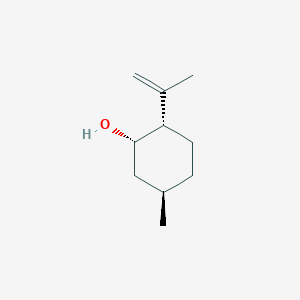 |
0.487 | D0S0AS | 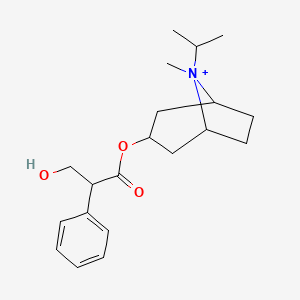 |
0.200 | ||
| ENC003125 | 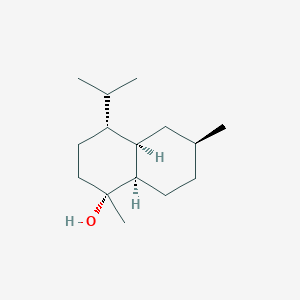 |
0.469 | D06PTA | 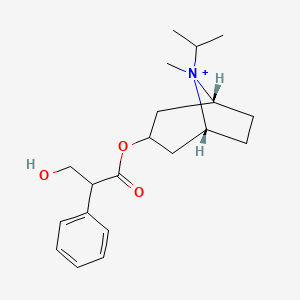 |
0.200 | ||
| ENC000791 | 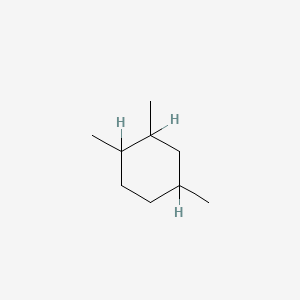 |
0.432 | D03DVJ | 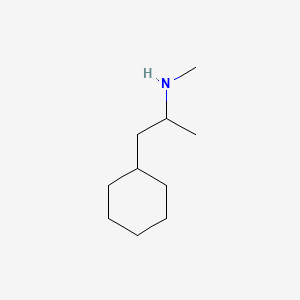 |
0.200 | ||
| ENC000762 | 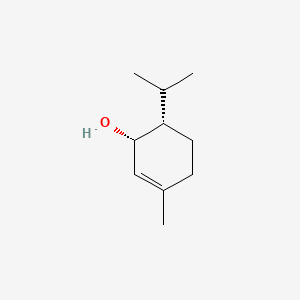 |
0.381 | D04SFH | 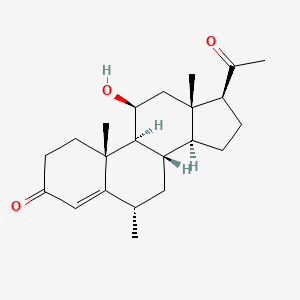 |
0.198 | ||
| ENC000763 | 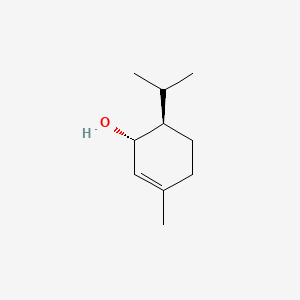 |
0.381 | D0N6FH | 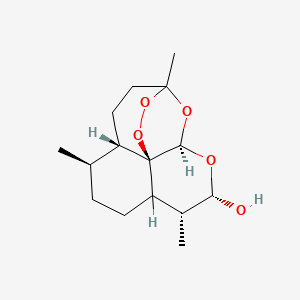 |
0.197 | ||
| ENC003266 |  |
0.378 | D07QKN | 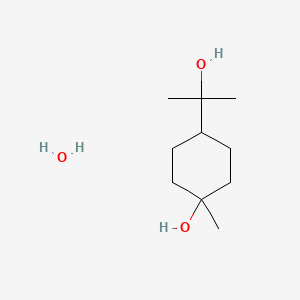 |
0.196 | ||
| ENC004915 | 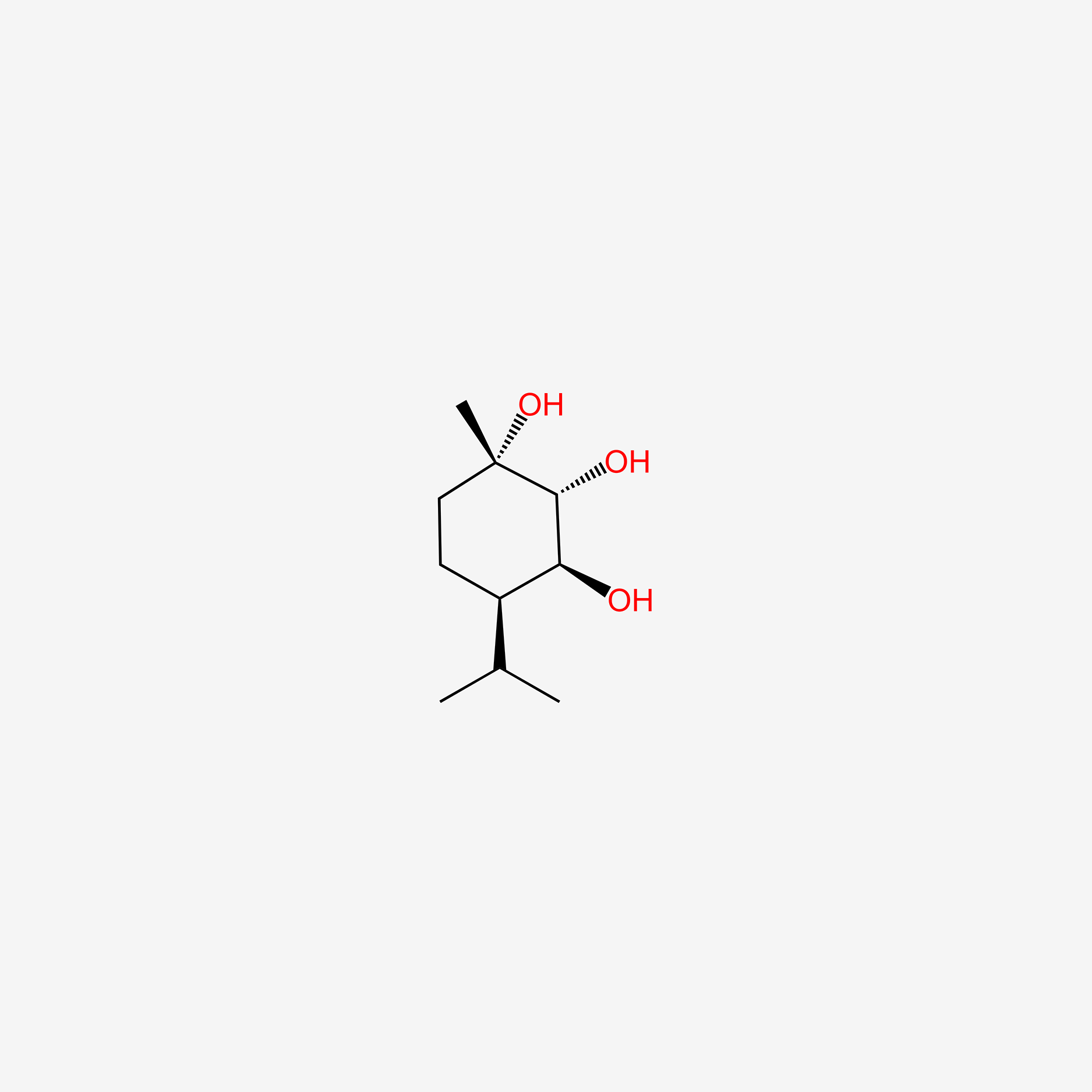 |
0.378 | D0R2KF | 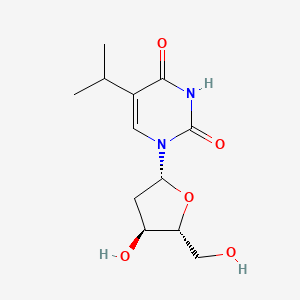 |
0.194 | ||
| ENC003087 | 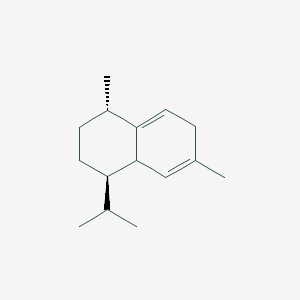 |
0.373 | D0G3SH | 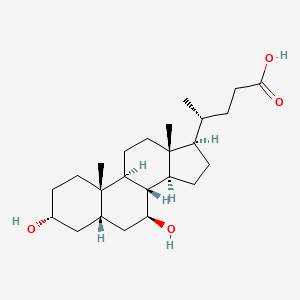 |
0.191 | ||