NPs Basic Information
|
Name |
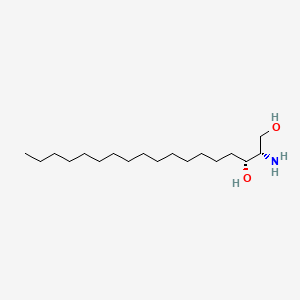
Sphinganine
|
| Molecular Formula | C18H39NO2 | |
| IUPAC Name* |
(2S,3R)-2-aminooctadecane-1,3-diol
|
|
| SMILES |
CCCCCCCCCCCCCCC[C@H]([C@H](CO)N)O
|
|
| InChI |
InChI=1S/C18H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h17-18,20-21H,2-16,19H2,1H3/t17-,18+/m0/s1
|
|
| InChIKey |
OTKJDMGTUTTYMP-ZWKOTPCHSA-N
|
|
| Synonyms |
Sphinganine; Octadecasphinganine; Dihydrosphingosine; D-erythro-Sphinganine; 764-22-7; C18-Dihydrosphingosine; D-erythro-Dihydrosphingosine; (2S,3R)-2-aminooctadecane-1,3-diol; D-erythro-2-Amino-1,3-octadecanediol; D-erythro-C18-Dihydrosphingosine; Dihydro-C18-sphingosine; D-erythro-1,3-Dihydroxy-2-aminooctadecane; erythro-Sphinganine; SPC 102860; C18-Sphingosine, dihydro-; 1,3-Octadecanediol, 2-amino-, (2S,3R)-; (2S,3R)-2-amino-1,3-octadecanediol; 1,3-Octadecanediol, 2-amino-, D-erythro-; (R-(R*,S*))-2-Aminooctadecane-1,3-diol; YT0ZSD64HM; 1,3-Octadecanediol, 2-amino-, (R-(R*,S*))-; CHEMBL448741; D-erythro-Dihydro-D-sphingosine; CHEBI:16566; 2-Amino-1,3-dihydroxyoctadecane; C18-sphinganine; D-erythro-C18-Dihydro-D-sphingosine; 1,3-Octadecanediol, 2-amino-, [R-(R*,S*)]-; d18:0; Sphinganine (d18:0); 3102-56-5; EINECS 212-116-0; UNII-YT0ZSD64HM; dihydro-D-erythrosphingosine; D-erythro-DHS; DIHYDROSPINGOSINE; Tocris-0749; C18-dihydro-Sphingosine; SPHINGANINE [INCI]; cid_3126; CBiol_002042; SCHEMBL40592; BSPBio_001288; KBioGR_000008; KBioSS_000008; Dihydro-D-erythro-Sphingosine; MLS001066334; (+)-erythro-dihydrosphingosine; BML3-D04; GTPL2453; Dihydrosphingosine (sphinganine); BCBcMAP01_000225; KBio2_000008; KBio2_002576; KBio2_005144; KBio3_000015; KBio3_000016; Bio1_000328; Bio1_000817; Bio1_001306; Bio2_000008; Bio2_000488; D-erythro-Sphinganine, C18 chain; DTXSID501016568; HMS1361A10; HMS1791A10; HMS1989A10; HMS2232G12; HMS3402A10; ZINC8036012; BDBM50244355; LMSP01020001; AKOS037645439; D-erythro-Dihydrosphingosine, >=98%; 2-amino-D-erythro-1,3-Octadecanediol; HY-W019838; IDI1_033758; NCGC00024767-01; NCGC00024767-02; NCGC00024767-03; NCGC00024767-04; AS-60774; SMR000471878; [R-(R*,S*)]-2-amino-1,3-Octadecanediol; CS-0031629; C00836; SR-01000597679; 1,3-Octadecanediol, 2-amino-, D-erythro- (8CI); Sphinganine (d18:0), D-erythro-sphinganine, powder; SR-01000597679-1; 1,3-Octadecanediol, 2-amino-, (2S,3R)- (9CI); Q27088847; 82E0B6E3-6AE1-425F-A39E-5BED56511028
|
|
| CAS | 764-22-7 | |
| PubChem CID | 91486 | |
| ChEMBL ID | CHEMBL448741 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 301.5 | ALogp: | 5.8 |
| HBD: | 3 | HBA: | 3 |
| Rotatable Bonds: | 16 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 66.5 | Aromatic Rings: | 0 |
| Heavy Atoms: | 21 | QED Weighted: | 0.361 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.153 | MDCK Permeability: | 0.00003040 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.969 |
| Human Intestinal Absorption (HIA): | 0.097 | 20% Bioavailability (F20%): | 0.606 |
| 30% Bioavailability (F30%): | 0.928 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.019 | Plasma Protein Binding (PPB): | 96.79% |
| Volume Distribution (VD): | 0.772 | Fu: | 2.46% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.344 | CYP1A2-substrate: | 0.23 |
| CYP2C19-inhibitor: | 0.248 | CYP2C19-substrate: | 0.058 |
| CYP2C9-inhibitor: | 0.09 | CYP2C9-substrate: | 0.378 |
| CYP2D6-inhibitor: | 0.592 | CYP2D6-substrate: | 0.489 |
| CYP3A4-inhibitor: | 0.261 | CYP3A4-substrate: | 0.066 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.351 | Half-life (T1/2): | 0.189 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.298 | Human Hepatotoxicity (H-HT): | 0.115 |
| Drug-inuced Liver Injury (DILI): | 0.043 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.063 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.943 | Carcinogencity: | 0.057 |
| Eye Corrosion: | 0.976 | Eye Irritation: | 0.936 |
| Respiratory Toxicity: | 0.945 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000781 | 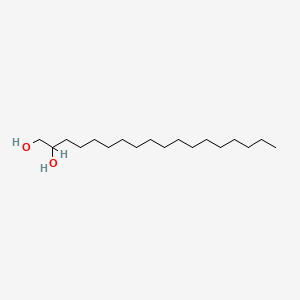 |
0.758 | D07ILQ |  |
0.587 | ||
| ENC001159 |  |
0.714 | D00AOJ |  |
0.537 | ||
| ENC000426 | 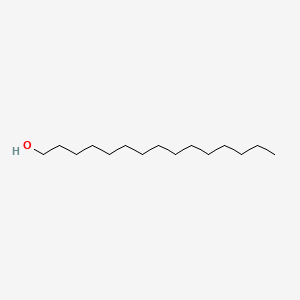 |
0.694 | D0Z5SM |  |
0.507 | ||
| ENC000515 | 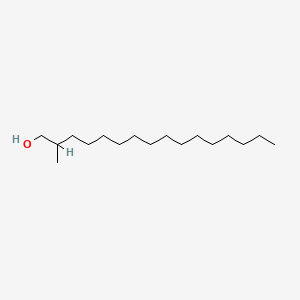 |
0.692 | D0O1PH |  |
0.471 | ||
| ENC000082 | 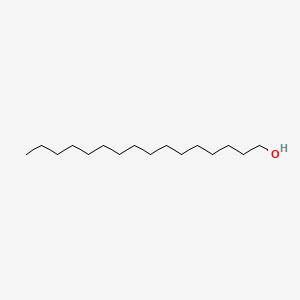 |
0.688 | D00FGR | 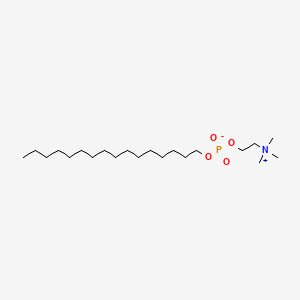 |
0.457 | ||
| ENC000489 | 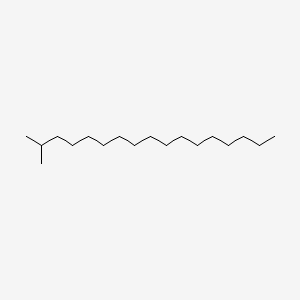 |
0.667 | D05ATI |  |
0.432 | ||
| ENC001528 | 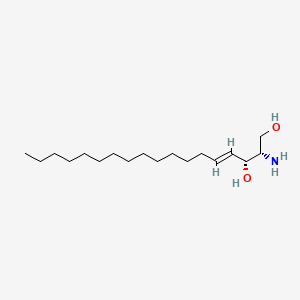 |
0.662 | D0P1RL |  |
0.383 | ||
| ENC000488 |  |
0.662 | D0T9TJ |  |
0.365 | ||
| ENC000486 |  |
0.657 | D00STJ | 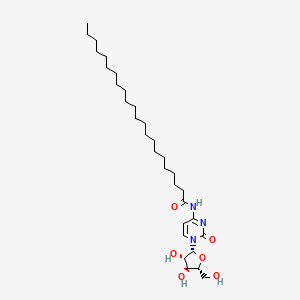 |
0.352 | ||
| ENC000666 | 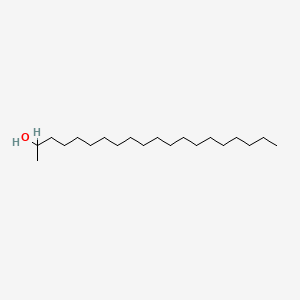 |
0.653 | D0XN8C |  |
0.311 | ||