NPs Basic Information
|
Name |
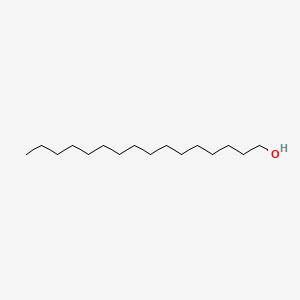
1-Hexadecanol
|
| Molecular Formula | C16H34O | |
| IUPAC Name* |
hexadecan-1-ol
|
|
| SMILES |
CCCCCCCCCCCCCCCCO
|
|
| InChI |
InChI=1S/C16H34O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h17H,2-16H2,1H3
|
|
| InChIKey |
BXWNKGSJHAJOGX-UHFFFAOYSA-N
|
|
| Synonyms |
1-Hexadecanol; cetyl alcohol; Hexadecan-1-ol; 36653-82-4; HEXADECANOL; Cetanol; Palmityl alcohol; Hexadecyl alcohol; N-Hexadecanol; n-1-Hexadecanol; n-Cetyl alcohol; Cetaffine; Cetylol; Cetal; Ethal; Ethol; Cetylic alcohol; n-Hexadecyl alcohol; Loxanwachs SK; Crodacol C; Loxanol K extra; 1-Hexadecyl alcohol; Elfacos C; Loxanol K; Crodacol-CAS; Crodacol-CAT; Atalco C; Cetalol CA; Siponol CC; Lanol C; 1-Cetanol; Hyfatol 16; Cachalot C-50; Cachalot C-51; Cachalot C-52; Alcohol C-16; Aldol 54; Dytol F-11; Adol; palmitic alcohol; Cyclal cetyl alcohol; Alfol 16; Lorol 24; Adol 52; Adol 54; Adol 52 NF; Hyfatol; Epal 16NF; 1-Hexadecyl alc; 16-Hexadecanol; C16 alcohol; Adol 520; n-Hexadecan-1-ol; Cetylalkohol; Isocetyl alcohol; 1-Hexanedecanol; FEMA No. 2554; Isohexadecyl alcohol; cetylalcohol; SSD RP; Normal primary hexadecyl alcohol; CO-1670; CO-1695; Cetyl alchol; Lipocol C; Fancol CA; Cetyl alcohol NF; Crodacol C70; Rita CA; 1-Hydroxyhexadecane; Cetanol (TN); Lanette 16; Philcohol 1600; Cetyl alcohol (NF); Cetyl alcohol [NF]; Lorol C16; LorolL 24; Cachalot C-50 NF; Adol 52NF; NSC-4194; 936JST6JCN; 67762-30-5; CHEBI:16125; NSC4194; 124-29-8; NCGC00159368-02; NCGC00159368-05; DSSTox_CID_7991; DSSTox_RID_78633; DSSTox_GSID_27991; Hexadecanol (VAN); Caswell No. 165D; FEMA Number 2554; Hexadecyl alcohol, normal; CAS-36653-82-4; HSDB 2643; NSC 4194; EINECS 253-149-0; UNII-936JST6JCN; EPA Pesticide Chemical Code 001508; Cetyl alcohol (hexadecanol); BRN 1748475; hexadecylalcohol; AI3-00755; Hexadecanol NF; Alcohol cetylicus; Ceraphyl ICA; Crodacol C95NF; Eutanol G16; Crodacol C95 NF; Laurex 16; MFCD00004760; Alfol 16RD; SSD (Salt/Mix); Cetanol (JP17); Epal 16; Hyfatol 16-95; Kalcol 6098; Loxiol VPG 1743; 1-Hexadecanol, 95%; SSD RP (Salt/Mix); CETANOL [JAN]; bmse000487; CHEMBL706; Michel XO-150-16; EC 253-149-0; 1-Hexadecanol, >=99%; CETYL ALCOHOL [II]; CETYL ALCOHOL [MI]; SCHEMBL3381; CETYL ALCOHOL [HSDB]; CETYL ALCOHOL [INCI]; 4-01-00-01876 (Beilstein Handbook Reference); 1-HEXADECANOL [FHFI]; CETYL ALCOHOL [VANDF]; CETYL ALCOHOL [MART.]; CETYL ALCOHOL [USP-RS]; CETYL ALCOHOL [WHO-DD]; CETYL ALCOHOL [WHO-IP]; DTXSID4027991; AMY6070; Cetyl alcohol, analytical standard; Cetyl alcohol, puriss., 95.0%; HMS3652H05; CS-D1348; HY-B1465; ZINC8214519; EINECS 252-964-9; Tox21_111609; Tox21_300325; CETYL ALCOHOL [EP MONOGRAPH]; LMFA05000061; s4173; STL283943; UNII-1800H64066; AKOS005287456; Tox21_111609_1; 1-Hexadecanol, ReagentPlus(R), 99%; CCG-266894; DB09494; ALCOHOL CETYLICUS [WHO-IP LATIN]; NCGC00159368-03; NCGC00159368-04; NCGC00159368-06; NCGC00254286-01; BS-16666; Cetyl alcohol, puriss., >=99.0% (GC); FT-0701357; FT-0707360; H0071; SW219201-1; Cetyl alcohol, SAJ special grade, >=98.0%; Cetyl alcohol, Selectophore(TM), >=99.0%; EN300-19351; 1-Hexadecanol, Vetec(TM) reagent grade, 94%; C00823; D00099; AB01566915_01; Q161632; SR-01000944409; SR-01000944409-1; 1800H64066; 810F139F-C57E-4DF1-916A-A320AD0DAF4D; F0001-1047; Z104473594; Cetyl alcohol, European Pharmacopoeia (EP) Reference Standard; Cetyl alcohol, United States Pharmacopeia (USP) Reference Standard; Cetyl Alcohol, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 36653-82-4 | |
| PubChem CID | 2682 | |
| ChEMBL ID | CHEMBL706 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 242.44 | ALogp: | 7.3 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 14 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 17 | QED Weighted: | 0.389 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.728 | MDCK Permeability: | 0.00001550 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.156 |
| 30% Bioavailability (F30%): | 0.992 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.129 | Plasma Protein Binding (PPB): | 97.41% |
| Volume Distribution (VD): | 2.697 | Fu: | 1.64% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.416 | CYP1A2-substrate: | 0.195 |
| CYP2C19-inhibitor: | 0.365 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.132 | CYP2C9-substrate: | 0.939 |
| CYP2D6-inhibitor: | 0.025 | CYP2D6-substrate: | 0.049 |
| CYP3A4-inhibitor: | 0.166 | CYP3A4-substrate: | 0.043 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.716 | Half-life (T1/2): | 0.147 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.227 | Human Hepatotoxicity (H-HT): | 0.012 |
| Drug-inuced Liver Injury (DILI): | 0.054 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.953 | Carcinogencity: | 0.043 |
| Eye Corrosion: | 0.993 | Eye Irritation: | 0.934 |
| Respiratory Toxicity: | 0.51 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000486 | 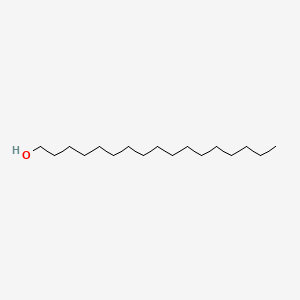 |
0.942 | D00AOJ |  |
0.731 | ||
| ENC000426 | 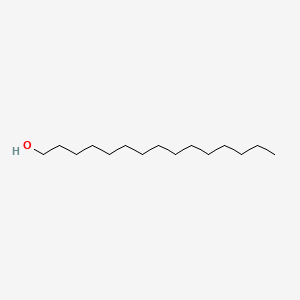 |
0.939 | D07ILQ |  |
0.703 | ||
| ENC000284 | 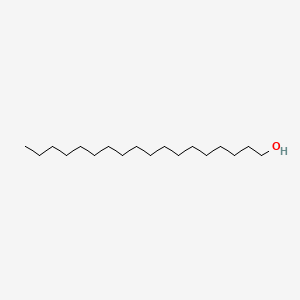 |
0.891 | D0Z5SM |  |
0.585 | ||
| ENC000745 |  |
0.845 | D00FGR |  |
0.550 | ||
| ENC000427 |  |
0.815 | D0O1PH |  |
0.513 | ||
| ENC000380 |  |
0.815 | D05ATI |  |
0.500 | ||
| ENC000431 |  |
0.803 | D00STJ |  |
0.393 | ||
| ENC000356 |  |
0.776 | D0P1RL |  |
0.379 | ||
| ENC000283 | 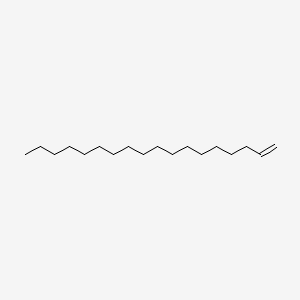 |
0.772 | D0MM8N | 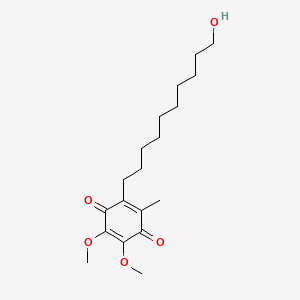 |
0.353 | ||
| ENC000400 | 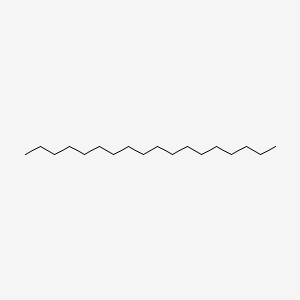 |
0.772 | D0T9TJ | 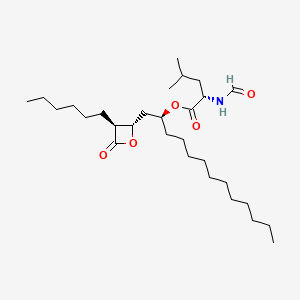 |
0.349 | ||