NPs Basic Information
|
Name |
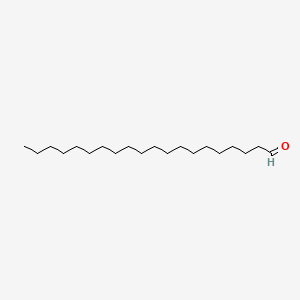
Icosanal
|
| Molecular Formula | C20H40O | |
| IUPAC Name* |
icosanal
|
|
| SMILES |
CCCCCCCCCCCCCCCCCCCC=O
|
|
| InChI |
InChI=1S/C20H40O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21/h20H,2-19H2,1H3
|
|
| InChIKey |
FWBUWJHWAKTPHI-UHFFFAOYSA-N
|
|
| Synonyms |
Icosanal; Eicosanal; 2400-66-0; ALDEHYDE C-20; 12001-36-4; Didecyl aldehyde; ZNJ1NXP04J; EINECS 219-275-5; UNII-ZNJ1NXP04J; AI3-24217; Eicosanal-; N-EICOSANAL; EICOSYL ALDEHYDE; 1-EICOSANAL; SCHEMBL154928; DTXSID00178733; CAA40066; LMFA06000250; ZINC59725627; AKOS032950020; CS-W004308; AS-56043; [(2,4-dimethylphenyl)amino](oxo)aceticacid; FT-0607716; H11190; J-521482; Q27295787
|
|
| CAS | 2400-66-0 | |
| PubChem CID | 75458 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 296.5 | ALogp: | 9.2 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 18 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 21 | QED Weighted: | 0.193 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.017 | MDCK Permeability: | 0.00000894 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.933 |
| 30% Bioavailability (F30%): | 1 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.263 | Plasma Protein Binding (PPB): | 97.45% |
| Volume Distribution (VD): | 3.897 | Fu: | 1.22% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.122 | CYP1A2-substrate: | 0.175 |
| CYP2C19-inhibitor: | 0.278 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.08 | CYP2C9-substrate: | 0.954 |
| CYP2D6-inhibitor: | 0.499 | CYP2D6-substrate: | 0.072 |
| CYP3A4-inhibitor: | 0.211 | CYP3A4-substrate: | 0.03 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.74 | Half-life (T1/2): | 0.076 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.47 | Human Hepatotoxicity (H-HT): | 0.009 |
| Drug-inuced Liver Injury (DILI): | 0.216 | AMES Toxicity: | 0.016 |
| Rat Oral Acute Toxicity: | 0.013 | Maximum Recommended Daily Dose: | 0.035 |
| Skin Sensitization: | 0.981 | Carcinogencity: | 0.071 |
| Eye Corrosion: | 0.995 | Eye Irritation: | 0.933 |
| Respiratory Toxicity: | 0.962 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000923 |  |
0.871 | D00AOJ |  |
0.707 | ||
| ENC000715 |  |
0.812 | D07ILQ |  |
0.571 | ||
| ENC000745 |  |
0.803 | D00FGR | 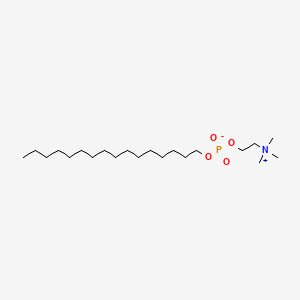 |
0.528 | ||
| ENC000285 | 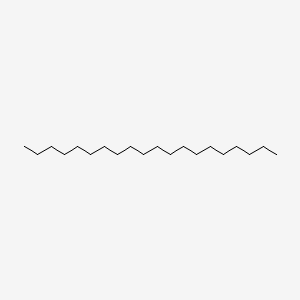 |
0.803 | D0Z5SM |  |
0.513 | ||
| ENC000357 | 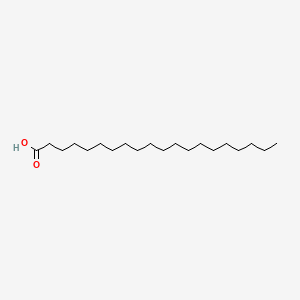 |
0.771 | D0O1PH |  |
0.477 | ||
| ENC000430 | 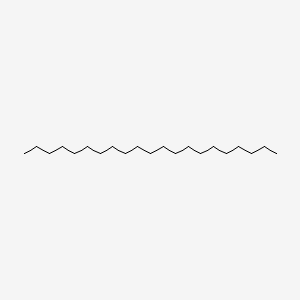 |
0.768 | D00STJ |  |
0.446 | ||
| ENC000431 |  |
0.768 | D05ATI |  |
0.440 | ||
| ENC000589 |  |
0.758 | D0T9TJ |  |
0.371 | ||
| ENC000429 |  |
0.758 | D0P1RL |  |
0.333 | ||
| ENC000428 |  |
0.758 | D00MLW |  |
0.318 | ||