NPs Basic Information
|
Name |
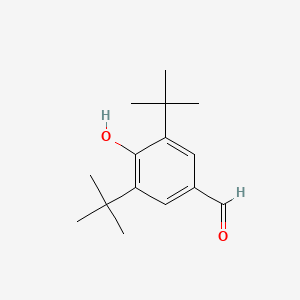
3,5-Di-tert-butyl-4-hydroxybenzaldehyde
|
| Molecular Formula | C15H22O2 | |
| IUPAC Name* |
3,5-ditert-butyl-4-hydroxybenzaldehyde
|
|
| SMILES |
CC(C)(C)C1=CC(=CC(=C1O)C(C)(C)C)C=O
|
|
| InChI |
InChI=1S/C15H22O2/c1-14(2,3)11-7-10(9-16)8-12(13(11)17)15(4,5)6/h7-9,17H,1-6H3
|
|
| InChIKey |
DOZRDZLFLOODMB-UHFFFAOYSA-N
|
|
| Synonyms |
3,5-Di-tert-butyl-4-hydroxybenzaldehyde; 1620-98-0; Benzaldehyde, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-; 3,5-ditert-butyl-4-hydroxybenzaldehyde; 3,5-Di-t-butyl-4-hydroxybenzaldehyde; Benzaldehyde, 3,5-di-tert-butyl-4-hydroxy-; MFCD00008826; 95VTI93VUL; 3,5-di(Tert-butyl)-4-hydroxybenzaldehyde; 3,5-bis(tert-butyl)-4-hydroxybenzaldehyde; 4-Formyl-2,6-di-tert-butylphenol; NSC-14450; 3,5-bis(1,1-dimethylethyl)-4-hydroxybenzaldehyde; EINECS 216-592-0; NSC 14450; UNII-95VTI93VUL; BRN 0982526; 3,5-Di-tert-butyl-4-hydroxy-benzaldehyde; 2,6-Di-tert-Butyl-4-formylphenol; NSC14450; BHT-OCH; 4-Hydroxy-3,5-di-tert-butylbenzaldehyde; DSSTox_CID_31447; DSSTox_RID_97333; DSSTox_GSID_57658; SCHEMBL85764; 4-08-00-00601 (Beilstein Handbook Reference); CHEMBL225623; DTXSID7057658; SCHEMBL22930710; ZINC56443; CHEBI:170060; ACT00782; BCP04892; Tox21_113770; 3,5-di-t-butyl4-hydroxybenzaldehyde; BBL010515; STK397393; AKOS000113376; CS-W012903; MS-3676; 3,5,-di-t-butyl-4-hydroxybenzaldehyde; 3,5-Di-t-butyl-4-hydroxy benzaldehyde; 3,5-ditert.butyl-4-hydroxybenzaldehyde; 3,5-di tert.butyl-4-hydroxybenzaldehyde; 3,5-di-tert-butyl 4-hydroxybenzaldehyde; 3,5-di-tert.butyl-4-hydroxybenzaldehyde; Benzaldehyde,5-di-tert-butyl-4-hydroxy-; NCGC00253643-01; NCGC00253643-02; AC-10539; 3,5- di-tert-butyl-4-hydroxybenzaldehyde; 3,5-Di-tert-butyl-4 -hydroxybenzaldehyde; 3,5-di-tert-butyl-4-hydrox-ybenzaldehyde; 3,5-di-tert.-butyl-4-hydroxybenzaldehyde; 3,5-di-tert.butyl-4-hydroxy benzaldehyde; 4-hydroxy-3,5-di-tert.-butylbenzaldehyde; CAS-1620-98-0; 3,5-Di-(tert-butyl)-4-hydroxybenzaldehyde; Benzaldehyde, 4-hydroxy-3,5-di-tert-butyl; DB-022118; 3,5-di-tertiary-butyl-4-hydroxybenzaldehyde; AM20050056; FT-0614732; EN300-17563; Benzoic aldehyde, 3,5-di-t-butyl-4-hydroxy-; AB01324450-02; 3,5-di0T0butyl-4-hydroxybenzaldehyde*hemihydrate; 620D980; A810339; AE-641/02487004; Benzaldehyde,5-bis(1,1-dimethylethyl)-4-hydroxy-; SR-01000944840; 3,5-Ditert-butyl-4-hydroxybenzaldehyde, AldrichCPR; SR-01000944840-1; W-107967; Q27271830; Z56957492; F1673-1281; 2,6-Ditert-butyl-4-(hydroxymethylene)-2,5-cyclohexadien-1-one #; 2,3,5,6-Detetrahydrocyclohexanone, 2,6-di-t-butyl-4-hydroxymethylene-; 3-TERT-BUTYL-2-HYDROXY-.BETA.,.BETA.,5-TRIMETHYLBENZENEETHYL-.BETA.-D-GLUCOSIDURONATE
|
|
| CAS | 1620-98-0 | |
| PubChem CID | 73219 | |
| ChEMBL ID | CHEMBL225623 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 234.33 | ALogp: | 4.4 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 17 | QED Weighted: | 0.728 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.793 | MDCK Permeability: | 0.00001380 |
| Pgp-inhibitor: | 0.825 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.153 | 20% Bioavailability (F20%): | 0.971 |
| 30% Bioavailability (F30%): | 0.89 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.784 | Plasma Protein Binding (PPB): | 97.53% |
| Volume Distribution (VD): | 2.807 | Fu: | 6.22% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.961 | CYP1A2-substrate: | 0.897 |
| CYP2C19-inhibitor: | 0.372 | CYP2C19-substrate: | 0.546 |
| CYP2C9-inhibitor: | 0.596 | CYP2C9-substrate: | 0.789 |
| CYP2D6-inhibitor: | 0.621 | CYP2D6-substrate: | 0.501 |
| CYP3A4-inhibitor: | 0.253 | CYP3A4-substrate: | 0.372 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.202 | Half-life (T1/2): | 0.361 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.007 | Human Hepatotoxicity (H-HT): | 0.026 |
| Drug-inuced Liver Injury (DILI): | 0.018 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.054 | Maximum Recommended Daily Dose: | 0.73 |
| Skin Sensitization: | 0.316 | Carcinogencity: | 0.03 |
| Eye Corrosion: | 0.951 | Eye Irritation: | 0.97 |
| Respiratory Toxicity: | 0.916 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000610 |  |
0.694 | D0H2DQ |  |
0.347 | ||
| ENC000725 |  |
0.694 | D0W7WC |  |
0.344 | ||
| ENC000079 | 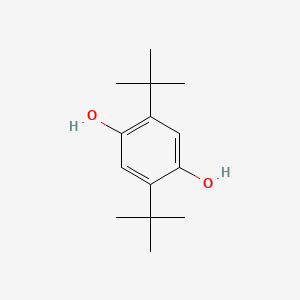 |
0.566 | D09EBS | 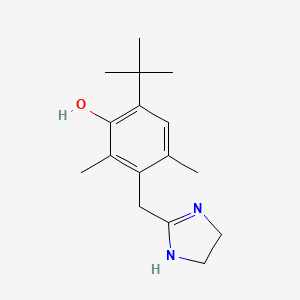 |
0.306 | ||
| ENC000346 | 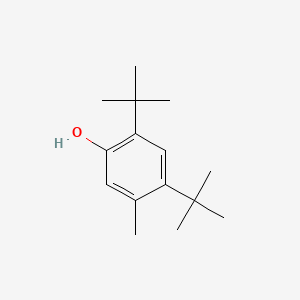 |
0.566 | D00NJL | 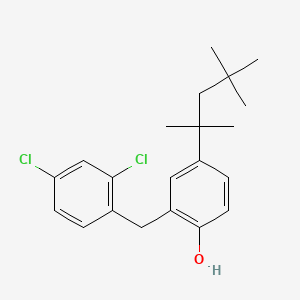 |
0.259 | ||
| ENC000658 | 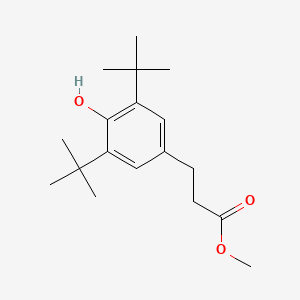 |
0.565 | D0E9CD |  |
0.259 | ||
| ENC000611 | 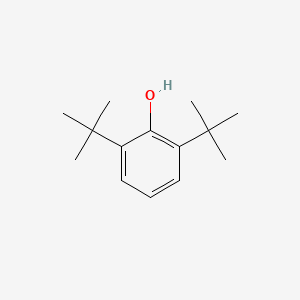 |
0.558 | D01JFT | 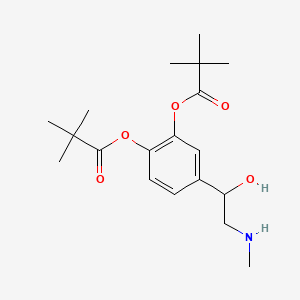 |
0.256 | ||
| ENC001398 | 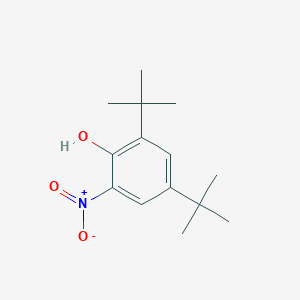 |
0.544 | D0Y4DY | 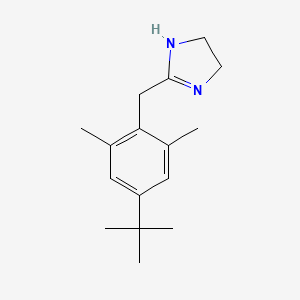 |
0.243 | ||
| ENC000185 | 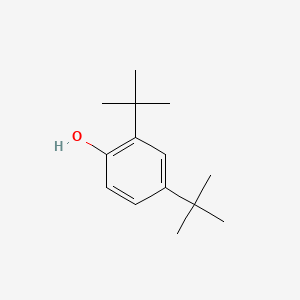 |
0.473 | D0M8RC | 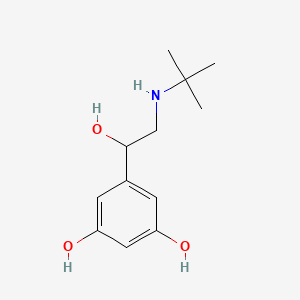 |
0.232 | ||
| ENC000744 | 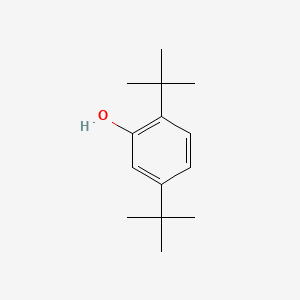 |
0.473 | D0X5NX | 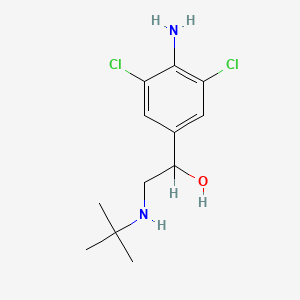 |
0.225 | ||
| ENC005113 | 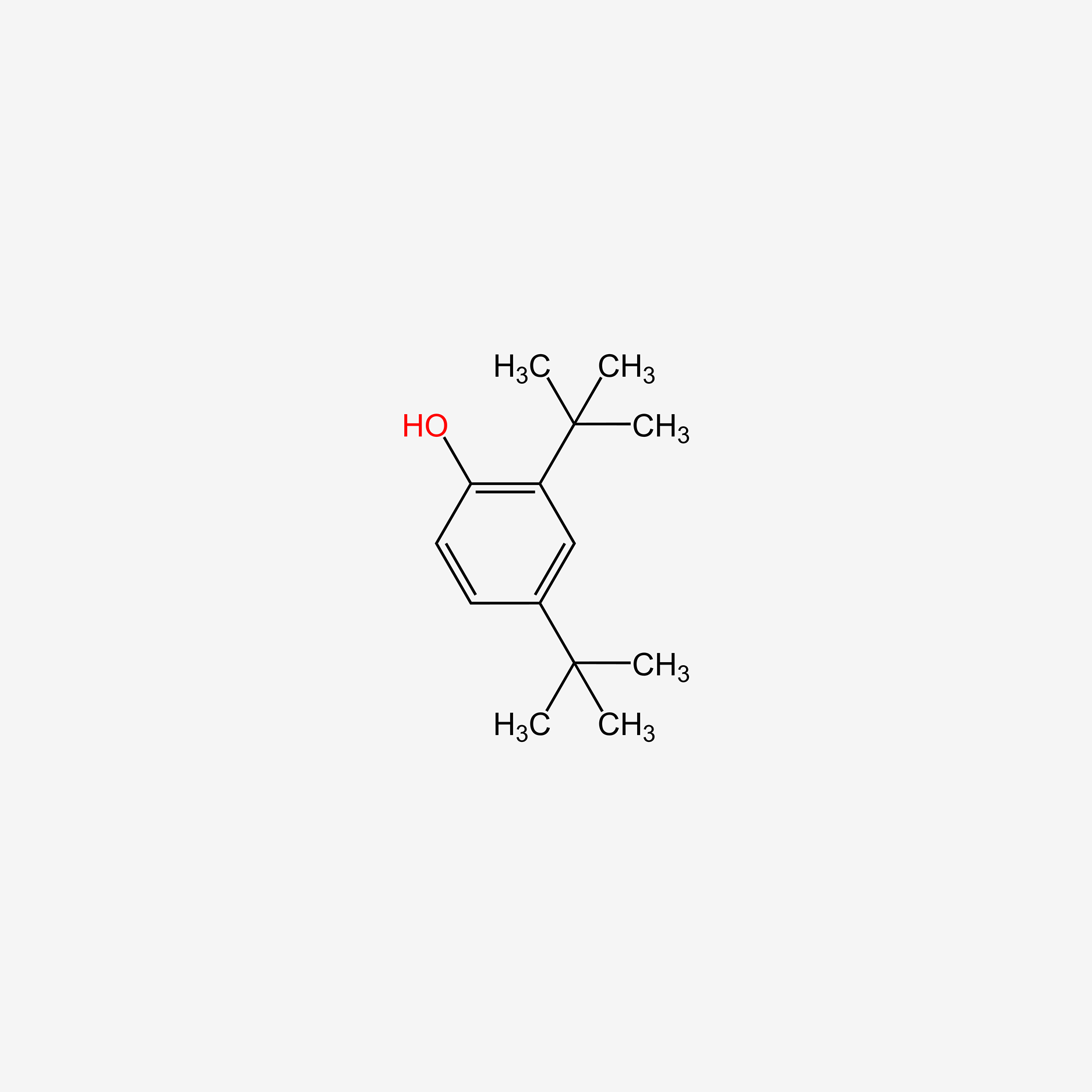 |
0.473 | D06YPU | 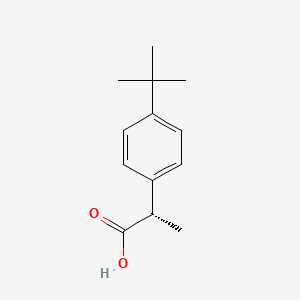 |
0.224 | ||