NPs Basic Information
|
Name |
Nobiletin
|
| Molecular Formula | C21H22O8 | |
| IUPAC Name* |
2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxychromen-4-one
|
|
| SMILES |
COC1=C(C=C(C=C1)C2=CC(=O)C3=C(O2)C(=C(C(=C3OC)OC)OC)OC)OC
|
|
| InChI |
InChI=1S/C21H22O8/c1-23-13-8-7-11(9-15(13)24-2)14-10-12(22)16-17(25-3)19(26-4)21(28-6)20(27-5)18(16)29-14/h7-10H,1-6H3
|
|
| InChIKey |
MRIAQLRQZPPODS-UHFFFAOYSA-N
|
|
| Synonyms |
Nobiletin; 478-01-3; Hexamethoxyflavone; 3',4',5,6,7,8-Hexamethoxyflavone; 5,6,7,8,3',4'-Hexamethoxyflavone; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxychromen-4-one; 2-(3,4-Dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-1-benzopyran-4-one; NSC-76751; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-chromen-4-one; D65ILJ7WLY; 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-; CHEMBL76447; Nobiletin (Hexamethoxyflavone); CHEBI:7602; NSC76751; NSC-618903; Flavone, 5,6,7,8,3',4'-hexamethoxy; SMR000156231; CCRIS 9012; UNII-D65ILJ7WLY; NSC 76751; MFCD03273560; CPD000156231; Nobiletin, >=97%; NOBILETIN [INCI]; Spectrum2_001697; Spectrum3_000921; Spectrum4_001020; NOBILETIN [USP-RS]; KBioGR_001519; MLS000574877; MLS000759462; MLS000877030; MLS001424129; Nobiletin, analytical standard; SCHEMBL244029; SPECTRUM1505268; SPBio_001654; MEGxp0_000930; ACon1_000921; KBio3_001922; DTXSID30197275; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-chromen-4-one; HMS2051D09; HMS2234A09; HMS3373C14; HMS3393D09; HMS3651G20; HEXAMETHOXYFLAVONE [WHO-DD]; HY-N0155; ZINC1531669; 3'4'5,6,7,8-Hexamethoxyflavone; BDBM50338976; CCG-38781; LMPK12111468; NSC618903; STL565829; AKOS015965334; NOBILETIN, 20% (Technical Grade); AC-1023; CS-5518; NC00186; SDCCGMLS-0066776.P001; NCGC00095703-01; NCGC00095703-02; NCGC00095703-06; NCGC00169228-01; 5,6,7,8,3'',4''-hexamethoxyflavone; AS-17452; NCI60_041691; DB-050181; FT-0686667; N0871; S2333; SW197566-2; A827343; FLAVONE, 3',4',5,6,7,8-HEXAMETHOXY-; SR-01000712262; Q-100511; Q2402963; SR-01000712262-5; BRD-K06753942-001-02-0; 2-(3,4-Dimethoxy-phenyl)-5,6,7,8-tetramethoxy-chromen-4-one; 4,5,6,7,8,9,10,11,12,13-decahydrocyclododeca[d]oxazole; 4H-1-Benzopyran-4-one,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-; 2-(3,4-Dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-1-benzopyran-4-one, 9CI; 3 inverted exclamation mark ,4 inverted exclamation mark ,5,6,7,8-HEXAMETHOXYFLAVONE
|
|
| CAS | 478-01-3 | |
| PubChem CID | 72344 | |
| ChEMBL ID | CHEMBL76447 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 402.4 | ALogp: | 3.0 |
| HBD: | 0 | HBA: | 8 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 81.7 | Aromatic Rings: | 3 |
| Heavy Atoms: | 29 | QED Weighted: | 0.58 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.518 | MDCK Permeability: | 0.00002380 |
| Pgp-inhibitor: | 0.999 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.013 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0.006 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.087 | Plasma Protein Binding (PPB): | 68.59% |
| Volume Distribution (VD): | 0.93 | Fu: | 30.21% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.2 | CYP1A2-substrate: | 0.988 |
| CYP2C19-inhibitor: | 0.518 | CYP2C19-substrate: | 0.904 |
| CYP2C9-inhibitor: | 0.619 | CYP2C9-substrate: | 0.913 |
| CYP2D6-inhibitor: | 0.002 | CYP2D6-substrate: | 0.922 |
| CYP3A4-inhibitor: | 0.533 | CYP3A4-substrate: | 0.846 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.605 | Half-life (T1/2): | 0.372 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.331 | Human Hepatotoxicity (H-HT): | 0.07 |
| Drug-inuced Liver Injury (DILI): | 0.915 | AMES Toxicity: | 0.202 |
| Rat Oral Acute Toxicity: | 0.326 | Maximum Recommended Daily Dose: | 0.02 |
| Skin Sensitization: | 0.23 | Carcinogencity: | 0.052 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.043 |
| Respiratory Toxicity: | 0.031 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000829 |  |
0.460 | D06GCK |  |
0.552 | ||
| ENC005880 |  |
0.445 | D02LZB |  |
0.422 | ||
| ENC001772 |  |
0.433 | D09DHY |  |
0.404 | ||
| ENC001751 |  |
0.431 | D0G4KG |  |
0.371 | ||
| ENC002760 |  |
0.421 | D01FFA |  |
0.351 | ||
| ENC001405 |  |
0.415 | D0NJ3V |  |
0.348 | ||
| ENC001571 |  |
0.398 | D0Y7TS | 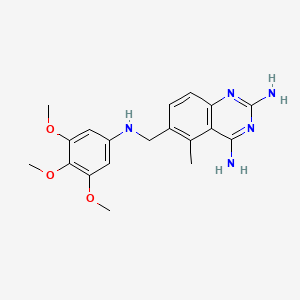 |
0.325 | ||
| ENC005522 | 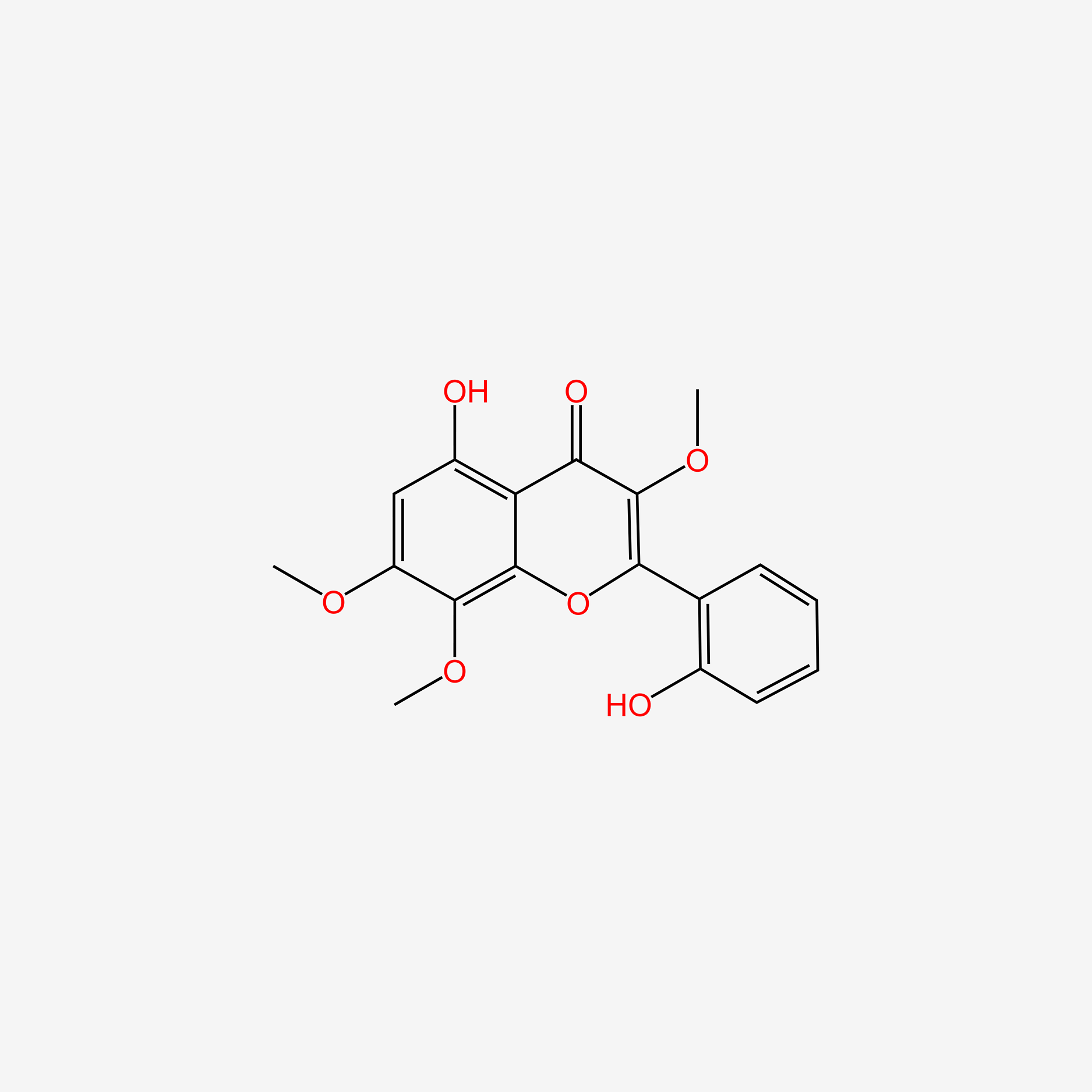 |
0.393 | D04TDQ | 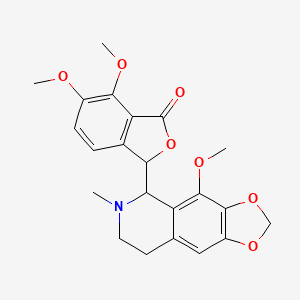 |
0.310 | ||
| ENC001403 | 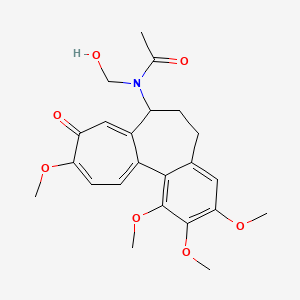 |
0.387 | D0R0FE | 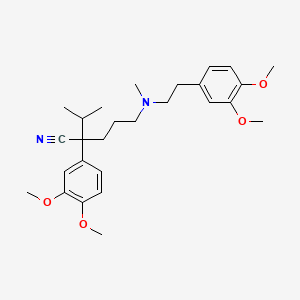 |
0.295 | ||
| ENC005936 | 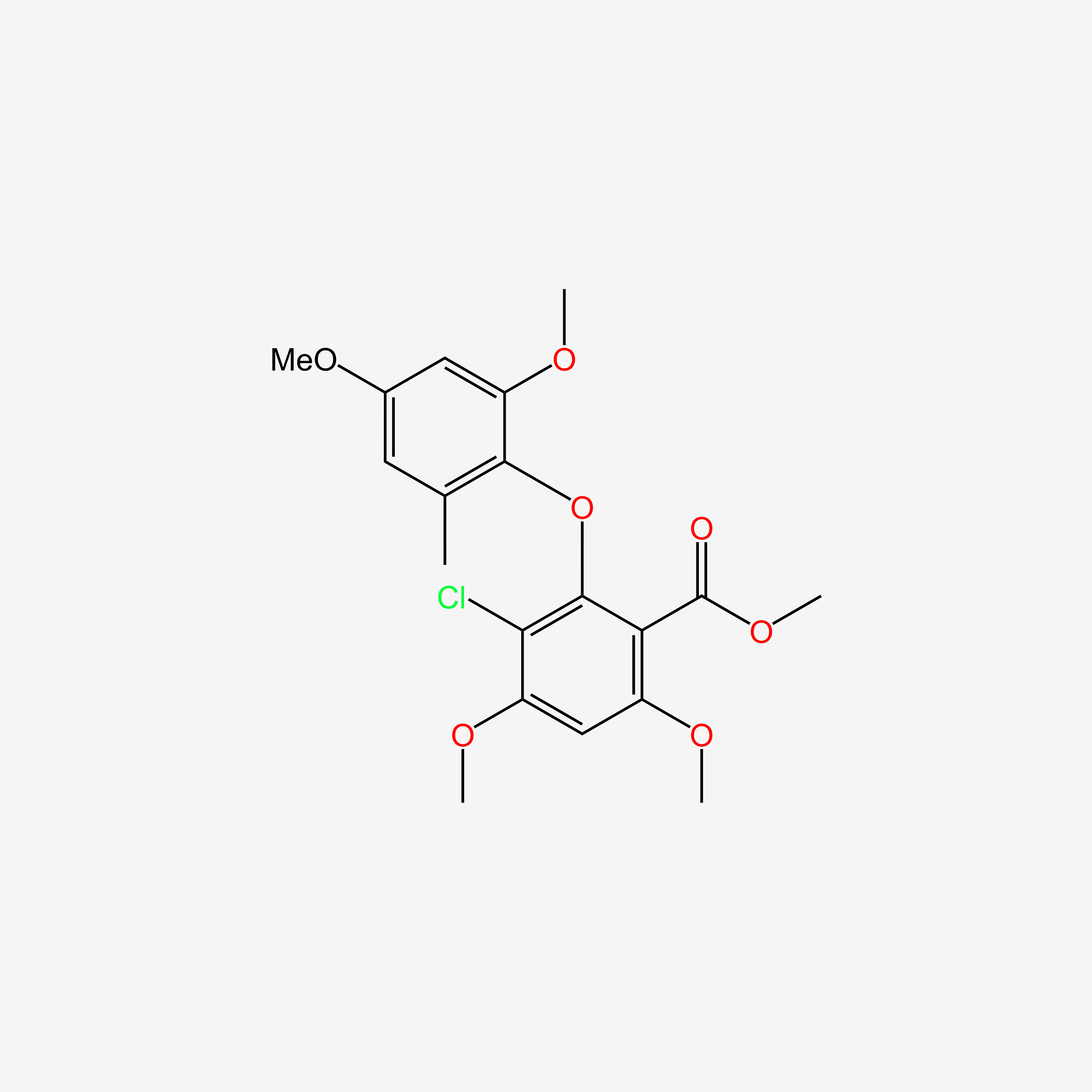 |
0.378 | D0A8FB | 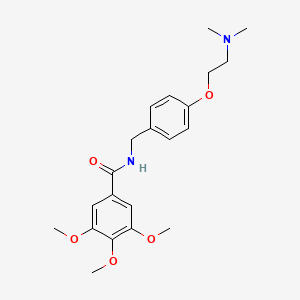 |
0.295 | ||