NPs Basic Information
|
Name |
Citromycetin
|
| Molecular Formula | C14H10O7 | |
| IUPAC Name* |
8,9-dihydroxy-2-methyl-4-oxo-5H-pyrano[3,2-c]chromene-10-carboxylic acid
|
|
| SMILES |
CC1=CC(=O)C2=C(O1)C3=C(C=C(C(=C3C(=O)O)O)O)OC2
|
|
| InChI |
InChI=1S/C14H10O7/c1-5-2-7(15)6-4-20-9-3-8(16)12(17)11(14(18)19)10(9)13(6)21-5/h2-3,16-17H,4H2,1H3,(H,18,19)
|
|
| InChIKey |
PKEPGKZPVDAVKI-UHFFFAOYSA-N
|
|
| Synonyms |
Citromycetin; 478-60-4; Frequentic acid; NSC53584; NSC 53584; NSC-53584; 6LQ9JP1YA7; 8,9-dihydroxy-2-methyl-4-oxo-5H-pyrano[3,2-c]chromene-10-carboxylic acid; 8,9-Dihydroxy-2-methyl-4-oxo-4H,5H-pyrano[3,2-c]chromene-10-carboxylic acid; 4H,5H-Pyrano[3,2-c][1]benzopyran-10-carboxylicacid, 8,9-dihydroxy-2-methyl-4-oxo-; 8,9-Dihydroxy-2-methyl-4-oxo-4H,5H-pyrano[3,2-c][1]benzopyran-10-carboxylic acid; UNII-6LQ9JP1YA7; BRN 0330020; 8,9-DIHYDROXY-2-METHYL-4-OXO-4H,5H-PYRANO(3,2-C)(1)BENZOPYRAN-10-CARBOXYLIC ACID; Spectrum_000290; SpecPlus_000355; Spectrum5_000777; CITROMYCETIN [MI]; KBioSS_000770; 4-19-00-03953 (Beilstein Handbook Reference); DivK1c_006451; SCHEMBL2137852; ZINC1165; ACon1_002335; KBio1_001395; KBio2_000770; KBio2_003338; KBio2_005906; DTXSID60197283; 4H,5H-Pyrano(3,2-c)(1)benzopyran-10-carboxylic acid, 8,9-dihydroxy-2-methyl-4-oxo-; NCGC00169943-01; HY-116479; CS-0065609; Q15410873; 4H,2-c][1]benzopyran-10-carboxylic acid, 8,9-dihydroxy-2-methyl-4-oxo-
|
|
| CAS | 478-60-4 | |
| PubChem CID | 65029 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 290.22 | ALogp: | 1.1 |
| HBD: | 3 | HBA: | 7 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 113.0 | Aromatic Rings: | 3 |
| Heavy Atoms: | 21 | QED Weighted: | 0.689 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.554 | MDCK Permeability: | 0.00000729 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.059 |
| Human Intestinal Absorption (HIA): | 0.188 | 20% Bioavailability (F20%): | 0.525 |
| 30% Bioavailability (F30%): | 0.995 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.029 | Plasma Protein Binding (PPB): | 88.95% |
| Volume Distribution (VD): | 0.742 | Fu: | 11.64% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.058 | CYP1A2-substrate: | 0.534 |
| CYP2C19-inhibitor: | 0.02 | CYP2C19-substrate: | 0.051 |
| CYP2C9-inhibitor: | 0.09 | CYP2C9-substrate: | 0.079 |
| CYP2D6-inhibitor: | 0.023 | CYP2D6-substrate: | 0.124 |
| CYP3A4-inhibitor: | 0.022 | CYP3A4-substrate: | 0.043 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.578 | Half-life (T1/2): | 0.894 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.532 |
| Drug-inuced Liver Injury (DILI): | 0.992 | AMES Toxicity: | 0.251 |
| Rat Oral Acute Toxicity: | 0.094 | Maximum Recommended Daily Dose: | 0.01 |
| Skin Sensitization: | 0.296 | Carcinogencity: | 0.151 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.032 |
| Respiratory Toxicity: | 0.531 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
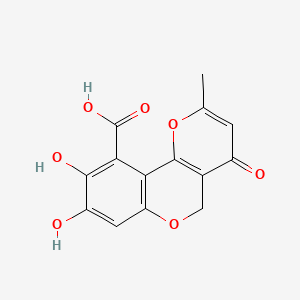
| ENC001505 | 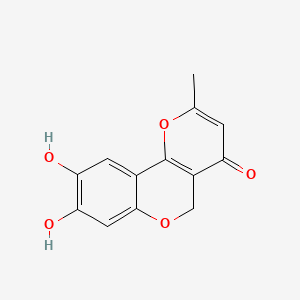 |
0.597 | D07UXP | 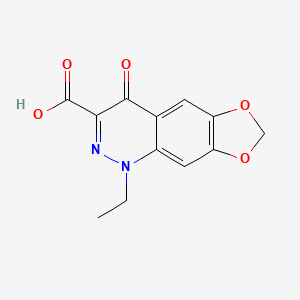 |
0.279 | ||
| ENC003632 | 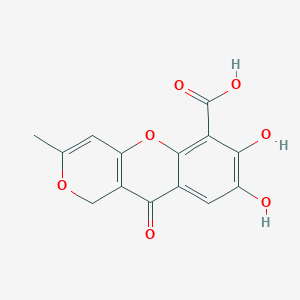 |
0.541 | D0K8KX |  |
0.272 | ||
| ENC004733 | 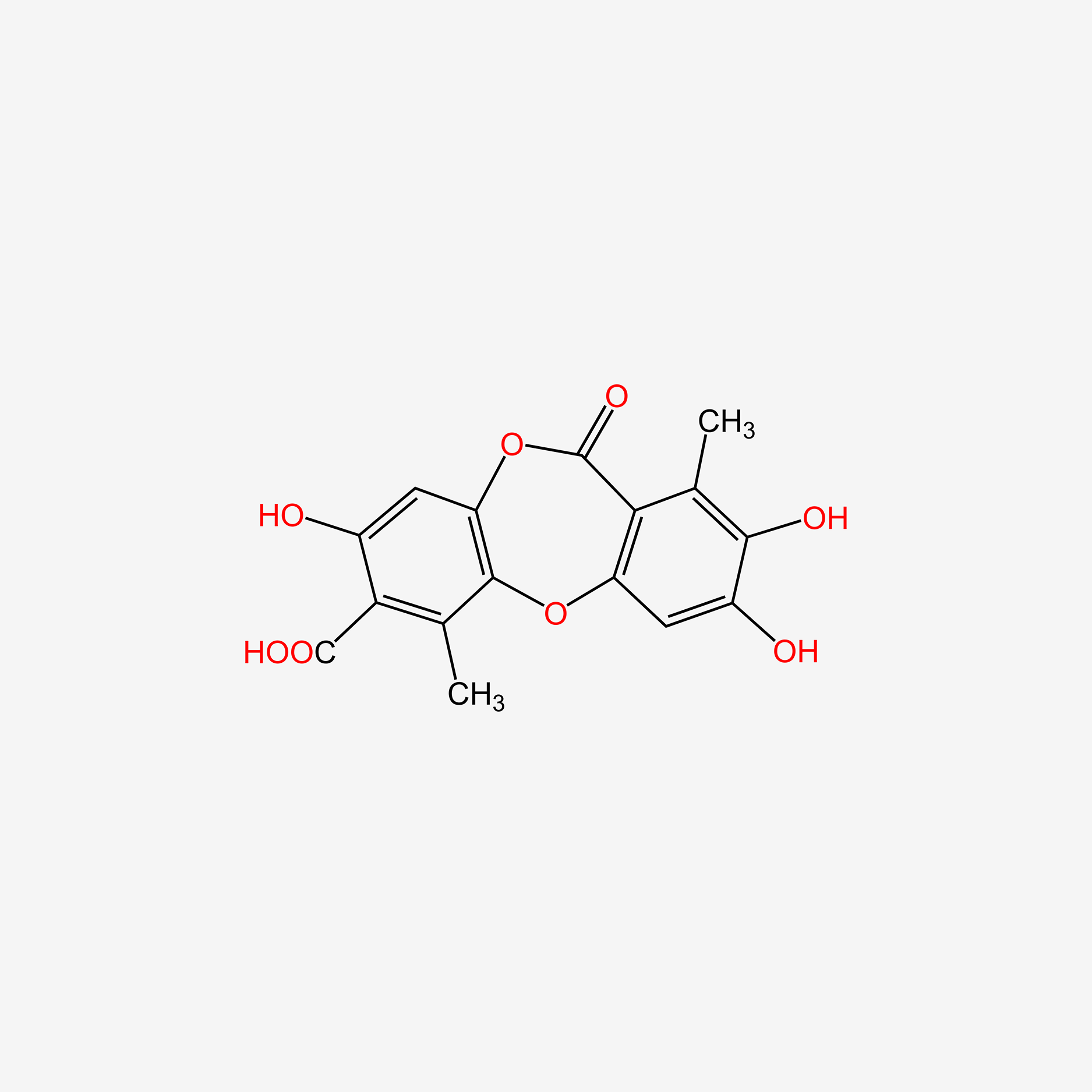 |
0.440 | D07MGA |  |
0.269 | ||
| ENC003635 | 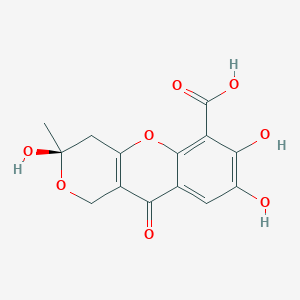 |
0.432 | D04AIT |  |
0.264 | ||
| ENC003720 | 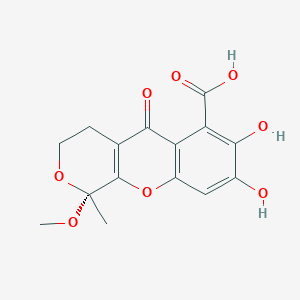 |
0.400 | D0Y7PG | 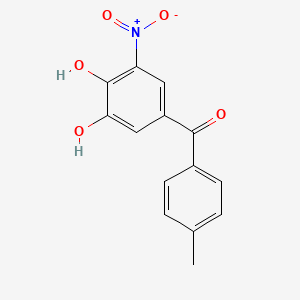 |
0.261 | ||
| ENC002865 | 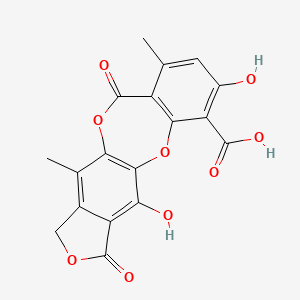 |
0.398 | D0FA2O | 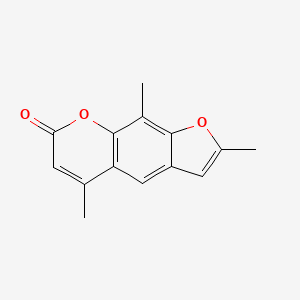 |
0.253 | ||
| ENC001518 | 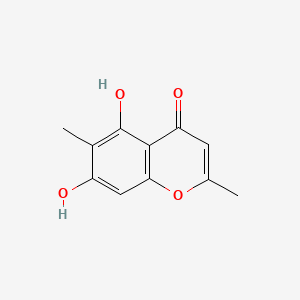 |
0.386 | D06FVX | 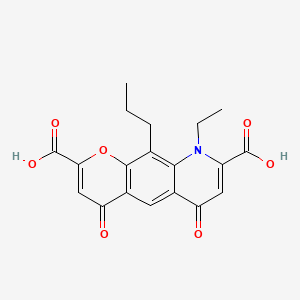 |
0.250 | ||
| ENC000935 | 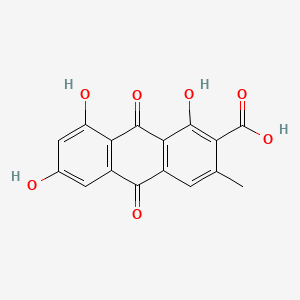 |
0.372 | D0G4KG | 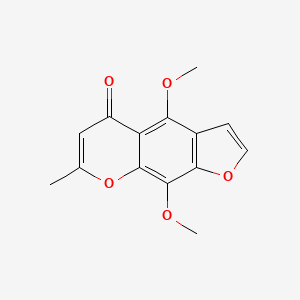 |
0.250 | ||
| ENC003772 | 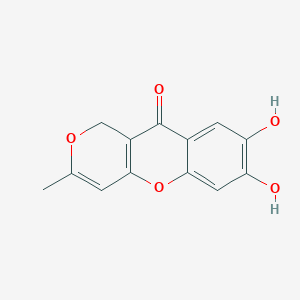 |
0.372 | D06GCK |  |
0.248 | ||
| ENC002811 | 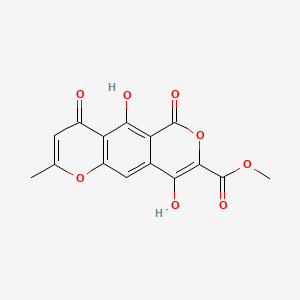 |
0.368 | D0G5UB | 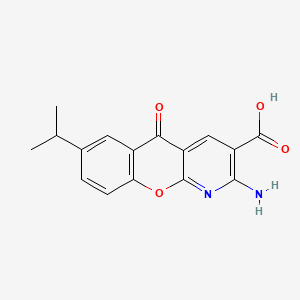 |
0.245 | ||