NPs Basic Information
|
Name |
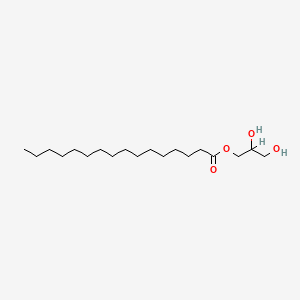
Glyceryl palmitate
|
| Molecular Formula | C19H38O4 | |
| IUPAC Name* |
2,3-dihydroxypropyl hexadecanoate
|
|
| SMILES |
CCCCCCCCCCCCCCCC(=O)OCC(CO)O
|
|
| InChI |
InChI=1S/C19H38O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19(22)23-17-18(21)16-20/h18,20-21H,2-17H2,1H3
|
|
| InChIKey |
QHZLMUACJMDIAE-UHFFFAOYSA-N
|
|
| Synonyms |
542-44-9; Monopalmitin; 1-Monopalmitin; 1-Palmitoyl-rac-glycerol; GLYCERYL PALMITATE; 2,3-dihydroxypropyl hexadecanoate; 1-Palmitoylglycerol; 2,3-Dihydroxypropyl palmitate; Glycerol 1-monopalmitate; Glycerol 1-palmitate; Glycerol 3-palmitate; 1-Monopalmitoylglycerol; Palmitin, 1-mono-; 19670-51-0; DL-alpha-Palmitin; alpha-Monopalmitin; Hexadecanoic acid, 2,3-dihydroxypropyl ester; 1-Monohexadecanoyl-rac-glycerol; .alpha.-Monopalmitin; L-alpha-Palmitin; 26657-96-5; Glycerides, C16-22; 1-hexadecanoyl-rac-glycerol; Palmitic acid .alpha.-monoglyceride; 1-Glyceryl monohexadecanoate; glyceryl 1-palmitate; Palmitic acid alpha-monoglyceride; Glycerol palmitate; 1-Glycerol hexadecanoate; Glyceryl monopalmitate; NSC 404240; MG(16:0/0:0/0:0)[rac]; U9H9OM3S75; CHEBI:69081; NSC404240; NSC-404240; 68002-70-0; 1-palmitoyl-glycerol; MG(16:0); monopalmitin-1-glyceride; 1-Monohexadecanoylglycerol; glycerol 1-monohexadecanoate; rac-1-Palmitoylglycerol; 3-Palmitoyl-rac-glycerol; rac-Glycerol 1-palmitate; Hexadecanoic acid, monoester with 1,2,3-propanetriol; UNII-U9H9OM3S75; Palmitin, 1-mono; (S)-2,3-Dihydroxypropyl palmitate; (1)-2,3-Dihydroxypropyl palmitate; EINECS 208-812-9; EINECS 243-218-3; EINECS 251-283-4; EINECS 268-083-8; D,L-alpha-Palmitin; (+-)-2,3-dihydroxypropyl palmitate; Glycerol monopalmitate; 1-hexadecanoylglycerol; rac-alpha-monopalmitin; rac-glyceryl palmitate; (+-)-2,3-Dihydroxypropyl hexadecanoate; 1-Monopalmitate Glycerol; (+-)-alpha-monopalmitin; (+-)-glyceryl palmitate; DL-1-MONOPALMITIN; (+/-)-1-monopalmitin; Glycerol .alpha.-palmitate; rac-1-monopalmitoylglycerol; EC 268-083-8; Glycerol alpha-Monopalmitate; rac-glycerol 1-monopalmitate; SCHEMBL74863; (+-)--glycerol 1-palmitate; (+-)-1-monopalmitoylglycerol; DL-alpha-Palmitin, >=99%; (+-)-glycerol 1-monopalmitate; CHEMBL1078140; CHEBI:75811; DTXSID00891470; rac-2,3-dihydroxypropyl palmitate; (.+/-.)-1-Hexadecanoylglycerol; AMY39945; BCP30618; MAG 16:0; LMGL01010001; MFCD00042734; rac-palmitic acid alpha-monoglyceride; rac-2,3-dihydroxypropyl hexadecanoate; AKOS015902112; (+/-)-1-HEXADECANOYLGLYCEROL; CCG-214232; SB48400; (+-)-palmitic acid alpha-monoglyceride; (+/-)-1-O-HEXADECANOYLGLYCEROL; Hexadecanoic acid,3-dihydroxypropyl ester; NCGC00186665-01; AS-49753; (.+/-.)-2,3-Dihydroxypropyl hexadecanoate; FT-0673487; FT-0673489; FT-0696902; FT-0699567; G0083; L10008; P-1125; A870508; SR-05000002594; J-012718; SR-05000002594-1; 2,3-Dihydroxypropyl hexadecanoate(Chunks or pellets); BRD-A80928489-001-01-0; Q27887716; 1-Monopalmitin;Glyceryl palmitate ;1-Palmitoyl-rac-glycerol;1-Palmitoylglycerol
|
|
| CAS | 542-44-9 | |
| PubChem CID | 14900 | |
| ChEMBL ID | CHEMBL1078140 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 330.5 | ALogp: | 6.3 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 18 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 66.8 | Aromatic Rings: | 0 |
| Heavy Atoms: | 23 | QED Weighted: | 0.294 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.815 | MDCK Permeability: | 0.00003200 |
| Pgp-inhibitor: | 0.062 | Pgp-substrate: | 0.862 |
| Human Intestinal Absorption (HIA): | 0.011 | 20% Bioavailability (F20%): | 0.926 |
| 30% Bioavailability (F30%): | 0.976 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.127 | Plasma Protein Binding (PPB): | 97.23% |
| Volume Distribution (VD): | 0.701 | Fu: | 1.67% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.511 | CYP1A2-substrate: | 0.169 |
| CYP2C19-inhibitor: | 0.306 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.294 | CYP2C9-substrate: | 0.887 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.054 |
| CYP3A4-inhibitor: | 0.403 | CYP3A4-substrate: | 0.063 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.172 | Half-life (T1/2): | 0.648 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.225 | Human Hepatotoxicity (H-HT): | 0.025 |
| Drug-inuced Liver Injury (DILI): | 0.029 | AMES Toxicity: | 0.023 |
| Rat Oral Acute Toxicity: | 0.019 | Maximum Recommended Daily Dose: | 0.011 |
| Skin Sensitization: | 0.931 | Carcinogencity: | 0.089 |
| Eye Corrosion: | 0.007 | Eye Irritation: | 0.134 |
| Respiratory Toxicity: | 0.329 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001700 | 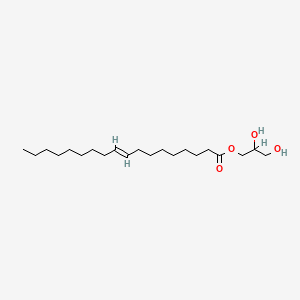 |
0.766 | D07ILQ |  |
0.667 | ||
| ENC000873 | 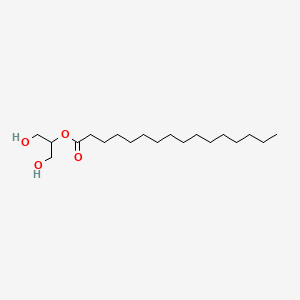 |
0.757 | D0O1PH |  |
0.523 | ||
| ENC005019 | 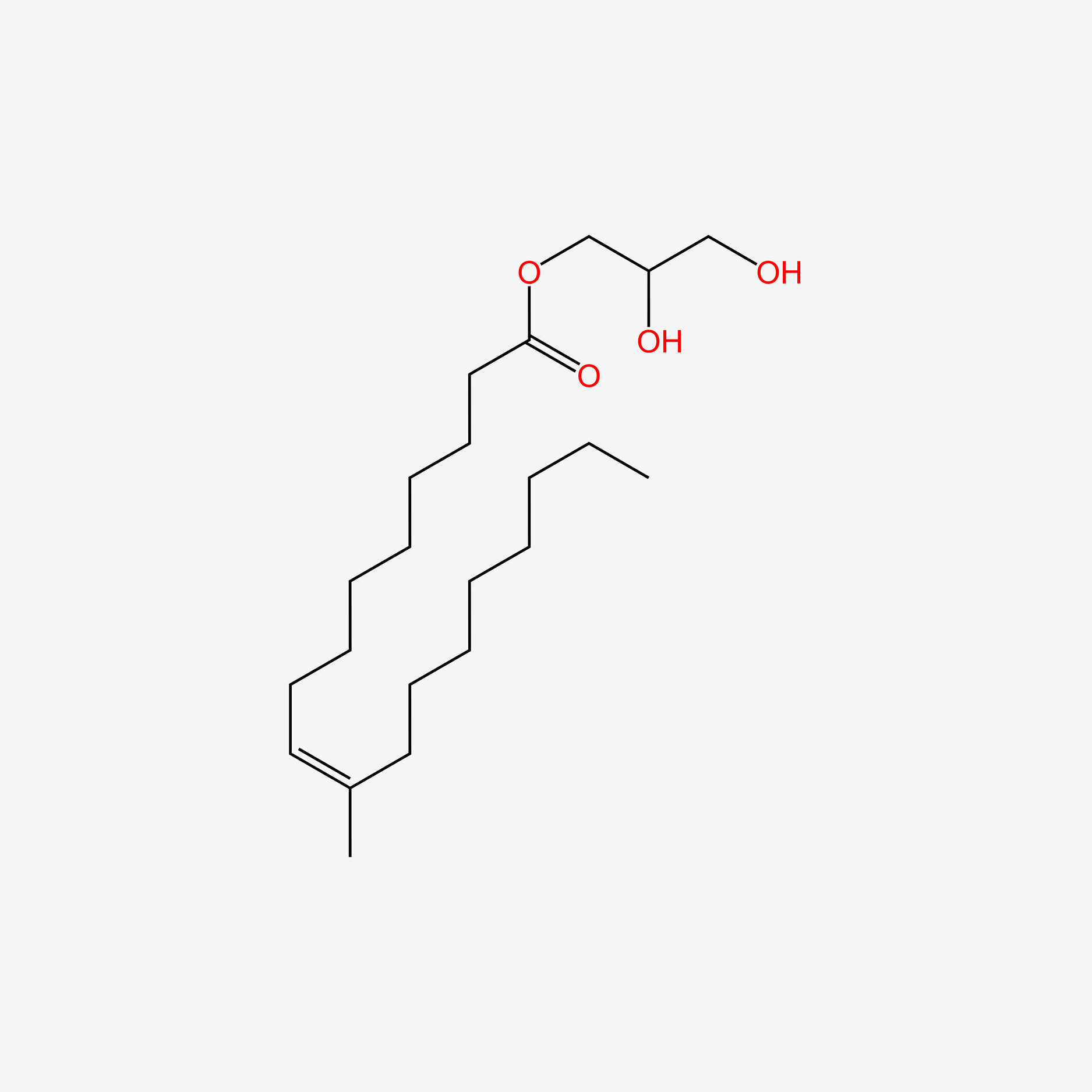 |
0.747 | D00AOJ |  |
0.517 | ||
| ENC000419 |  |
0.743 | D0Z5SM |  |
0.506 | ||
| ENC000575 |  |
0.736 | D00FGR |  |
0.474 | ||
| ENC000781 |  |
0.718 | D00MLW | 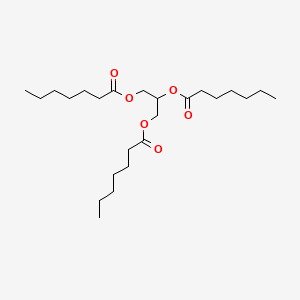 |
0.447 | ||
| ENC000258 | 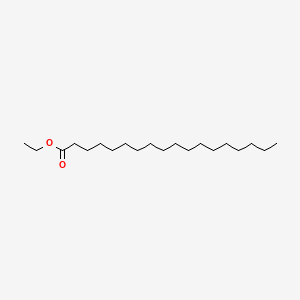 |
0.707 | D05ATI |  |
0.436 | ||
| ENC000271 | 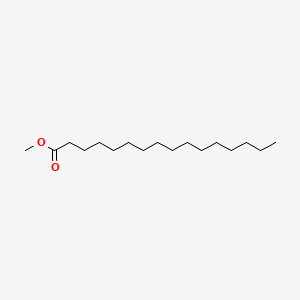 |
0.700 | D00STJ | 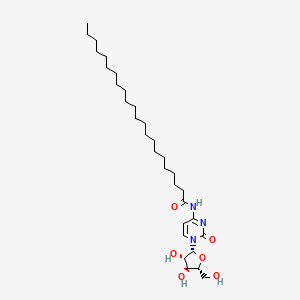 |
0.398 | ||
| ENC000496 | 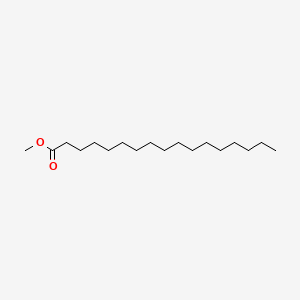 |
0.694 | D0Z1QC | 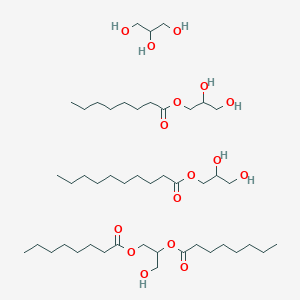 |
0.382 | ||
| ENC001234 | 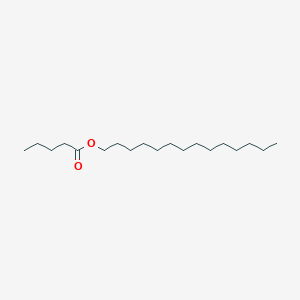 |
0.689 | D0P1RL | 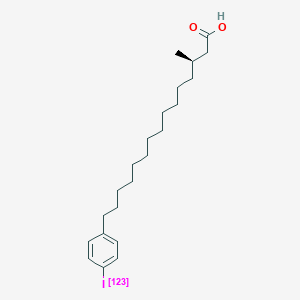 |
0.374 | ||