NPs Basic Information
|
Name |
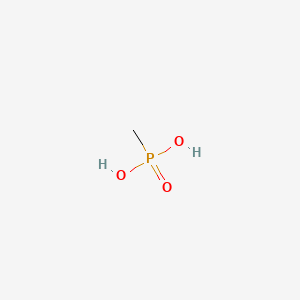
Methylphosphonic acid
|
| Molecular Formula | CH5O3P | |
| IUPAC Name* |
methylphosphonic acid
|
|
| SMILES |
CP(=O)(O)O
|
|
| InChI |
InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4)
|
|
| InChIKey |
YACKEPLHDIMKIO-UHFFFAOYSA-N
|
|
| Synonyms |
Methylphosphonic acid; 993-13-5; Methanephosphonic acid; Phosphonic acid, methyl-; METHYL PHOSPHONIC ACID; Phosphonic acid, P-methyl-; CHEMBL122938; CHEBI:45129; 329W4YM10Z; NSC-119358; methyl phosphonate; MFCD00002137; PHOSPHONOMETHYL GROUP; UNII-329W4YM10Z; HSDB 6762; VXA; EINECS 213-607-2; NSC 119358; Methyl-phosphonic acid; p-methylphosphonic acid; AI3-51156; EC 213-607-2; Methylphosphonic acid, 98%; DTXSID5047748; DIHYDROGEN METHYLPHOSPHONATE; BDBM50131862; NSC119358; ZINC84544184; METHYL PHOSPHONIC ACID [HSDB]; AKOS017343694; DB-009772; AM20120404; FT-0628284; Methylphosphonic acid, 99.0-101.0% (T); C20396; D95909; L000171; J-522698; Q15634110; 57H
|
|
| CAS | 993-13-5 | |
| PubChem CID | 13818 | |
| ChEMBL ID | CHEMBL122938 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 96.022 | ALogp: | -1.6 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.5 | Aromatic Rings: | 0 |
| Heavy Atoms: | 5 | QED Weighted: | 0.424 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.922 | MDCK Permeability: | 0.00170799 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.514 | 20% Bioavailability (F20%): | 0.144 |
| 30% Bioavailability (F30%): | 0.889 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.35 | Plasma Protein Binding (PPB): | 14.69% |
| Volume Distribution (VD): | 0.967 | Fu: | 86.42% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.008 | CYP1A2-substrate: | 0.429 |
| CYP2C19-inhibitor: | 0.034 | CYP2C19-substrate: | 0.057 |
| CYP2C9-inhibitor: | 0.006 | CYP2C9-substrate: | 0.692 |
| CYP2D6-inhibitor: | 0.013 | CYP2D6-substrate: | 0.135 |
| CYP3A4-inhibitor: | 0.004 | CYP3A4-substrate: | 0.049 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.081 | Half-life (T1/2): | 0.742 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.005 | Human Hepatotoxicity (H-HT): | 0.03 |
| Drug-inuced Liver Injury (DILI): | 0.023 | AMES Toxicity: | 0.037 |
| Rat Oral Acute Toxicity: | 0.009 | Maximum Recommended Daily Dose: | 0.526 |
| Skin Sensitization: | 0.568 | Carcinogencity: | 0.034 |
| Eye Corrosion: | 0.989 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.503 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001064 | 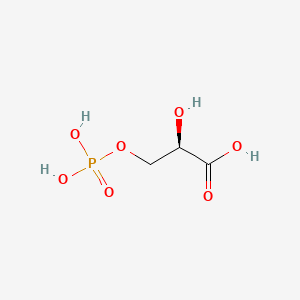 |
0.233 | D01GYT | 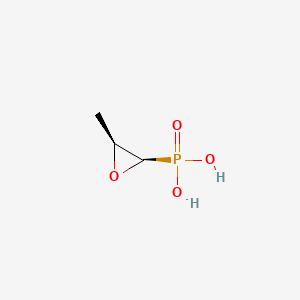 |
0.381 | ||
| ENC000037 | 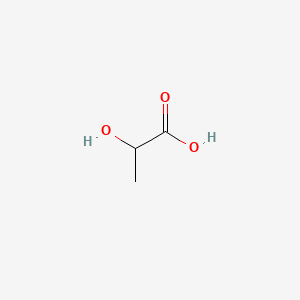 |
0.211 | D00NNC |  |
0.296 | ||
| ENC000009 |  |
0.200 | D0I6JF |  |
0.294 | ||
| ENC000046 |  |
0.182 | D0B4KH | 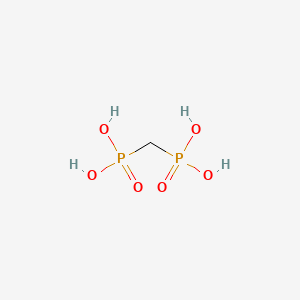 |
0.292 | ||
| ENC000469 | 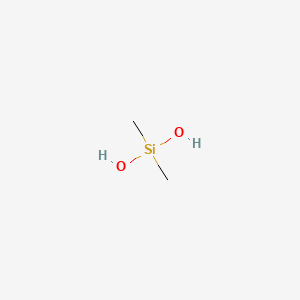 |
0.176 | D0DK8G | 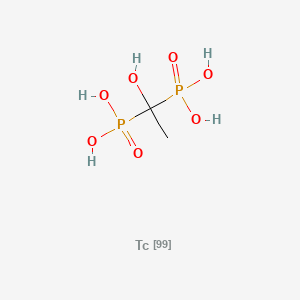 |
0.286 | ||
| ENC000568 | 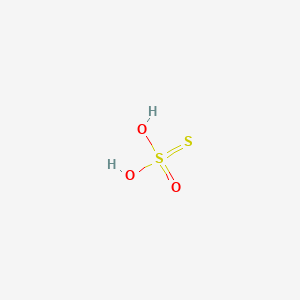 |
0.176 | D00XUN | 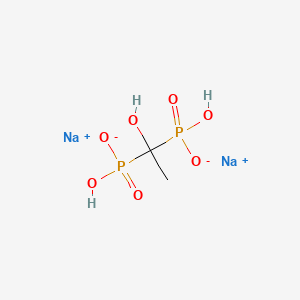 |
0.276 | ||
| ENC000031 | 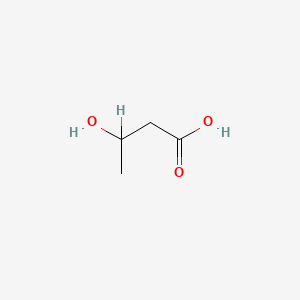 |
0.174 | D00HNB | 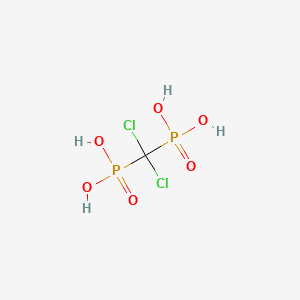 |
0.250 | ||
| ENC000288 | 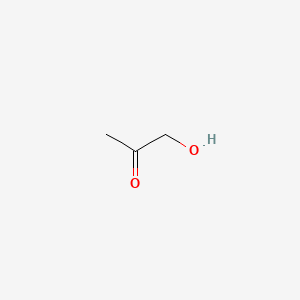 |
0.167 | D09UXE |  |
0.226 | ||
| ENC000057 |  |
0.167 | D0BF8G |  |
0.206 | ||
| ENC000058 |  |
0.167 | D08QGD |  |
0.200 | ||