NPs Basic Information
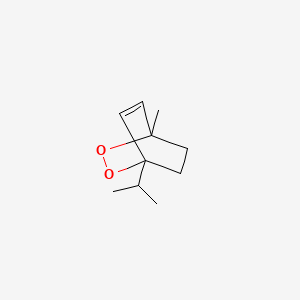
|
Name |
Ascaridole
|
| Molecular Formula | C10H16O2 | |
| IUPAC Name* |
1-methyl-4-propan-2-yl-2,3-dioxabicyclo[2.2.2]oct-5-ene
|
|
| SMILES |
CC(C)C12CCC(C=C1)(OO2)C
|
|
| InChI |
InChI=1S/C10H16O2/c1-8(2)10-6-4-9(3,5-7-10)11-12-10/h4,6,8H,5,7H2,1-3H3
|
|
| InChIKey |
MGYMHQJELJYRQS-UHFFFAOYSA-N
|
|
| Synonyms |
ASCARIDOLE; Ascaridol; 512-85-6; Ascaricum; Ascarisin; Askaridol; Ascaridiol; 1,4-Peroxido-p-menthene-2; 1,4-Peroxy-p-menth-2-ene; 1,4-Epidioxy-p-menth-2-ene; cis-Ascaridole; 1,4-Epidioxy-2-p-menthene; 2,3-Dioxabicyclo[2.2.2]oct-5-ene, 1-methyl-4-(1-methylethyl)-; CHEBI:2866; 1-Methyl-4-(1-methylethyl)-2,3-dioxabicyclo(2.2.2)oct-5-ene; 1-methyl-4-(1-methylethyl)-2,3-dioxabicyclo[2.2.2]oct-5-ene; 2,3-Dioxabicyclo(2.2.2)oct-5-ene, 1-methyl-4-(1-methylethyl)-; NSC 406266; 2, 1-isopropyl-4-methyl-; p-Menth-2-ene,4-epidioxy-; 1-Isopropyl-4-methyl-2,3-dioxabicyclo[2.2.2]oct-5-ene; 1-methyl-4-(propan-2-yl)-2,3-dioxabicyclo[2.2.2]oct-5-ene; 4-methyl-1-(propan-2-yl)-2,3-dioxabicyclo[2.2.2]oct-5-ene; 2, 1-methyl-4-(1-methylethyl)-; WLN: T66 A B AO BO DUTJ CY F; 2,3-Dioxabicyclo(2.2.2)oct-5-ene, 1-isopropyl-4-methyl-; Ascaridole (organic peroxide) [Forbidden]; 1-Isopropyl-4-methyl-2,3-Dioxabicyclo(2.2.2)oct-5-ene; Uncinacina; Ascapurin; NSC-406266; NSC-406924; Kebal II; 1-methyl-4-propan-2-yl-2,3-dioxabicyclo[2.2.2]oct-5-ene; SCHEMBL156673; Discontiuned Unable to ship""; 1, 4-Peroxy-p-menth-2-ene; CHEMBL467614; 1, 4-Epidioxy-p-menth-2-ene; NSC406266; NSC406924; AKOS004910035; FT-0662302; C09836; Q419442; 1-isopropyl-4-methyl-7-oxabicyclo[2.2.1]hept-2-ene; 1-Isopropyl-4-methyl-2,3-dioxabicyclo[2.2.2]oct-5-ene #; 1-Methyl-4-(1-methylethyl)-2,3-dioxabicyclo[2.2.2]oct-5-ene, 9CI
|
|
| CAS | 512-85-6 | |
| PubChem CID | 10545 | |
| ChEMBL ID | CHEMBL467614 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 168.23 | ALogp: | 2.3 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 18.5 | Aromatic Rings: | 3 |
| Heavy Atoms: | 12 | QED Weighted: | 0.442 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.298 | MDCK Permeability: | 0.00002650 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.02 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.131 | Plasma Protein Binding (PPB): | 80.00% |
| Volume Distribution (VD): | 1.556 | Fu: | 27.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.107 | CYP1A2-substrate: | 0.87 |
| CYP2C19-inhibitor: | 0.113 | CYP2C19-substrate: | 0.943 |
| CYP2C9-inhibitor: | 0.074 | CYP2C9-substrate: | 0.202 |
| CYP2D6-inhibitor: | 0.06 | CYP2D6-substrate: | 0.762 |
| CYP3A4-inhibitor: | 0.44 | CYP3A4-substrate: | 0.609 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.372 | Half-life (T1/2): | 0.525 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.022 | Human Hepatotoxicity (H-HT): | 0.256 |
| Drug-inuced Liver Injury (DILI): | 0.114 | AMES Toxicity: | 0.152 |
| Rat Oral Acute Toxicity: | 0.073 | Maximum Recommended Daily Dose: | 0.037 |
| Skin Sensitization: | 0.561 | Carcinogencity: | 0.884 |
| Eye Corrosion: | 0.098 | Eye Irritation: | 0.955 |
| Respiratory Toxicity: | 0.283 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001292 | 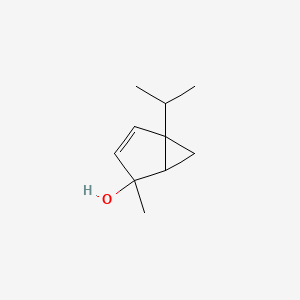 |
0.319 | D08KVZ | 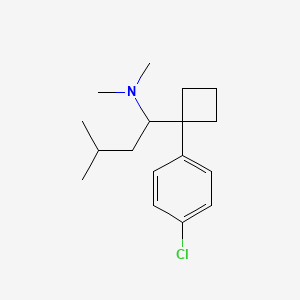 |
0.183 | ||
| ENC000331 | 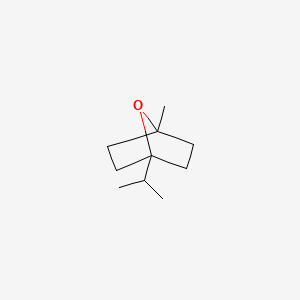 |
0.313 | D01CKY | 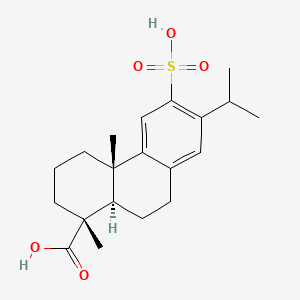 |
0.172 | ||
| ENC002232 | 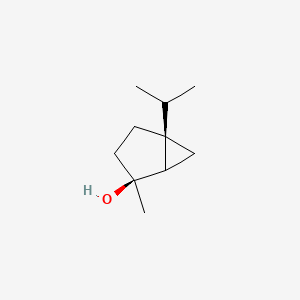 |
0.292 | D0K7LU | 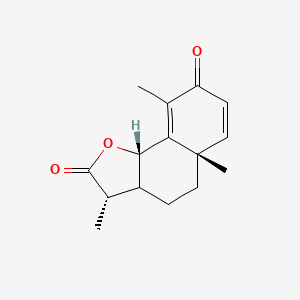 |
0.171 | ||
| ENC002264 | 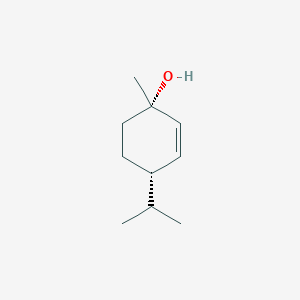 |
0.292 | D0N6FH |  |
0.171 | ||
| ENC000872 |  |
0.292 | D0S3WH |  |
0.171 | ||
| ENC000653 |  |
0.292 | D0H1QY |  |
0.170 | ||
| ENC005252 | 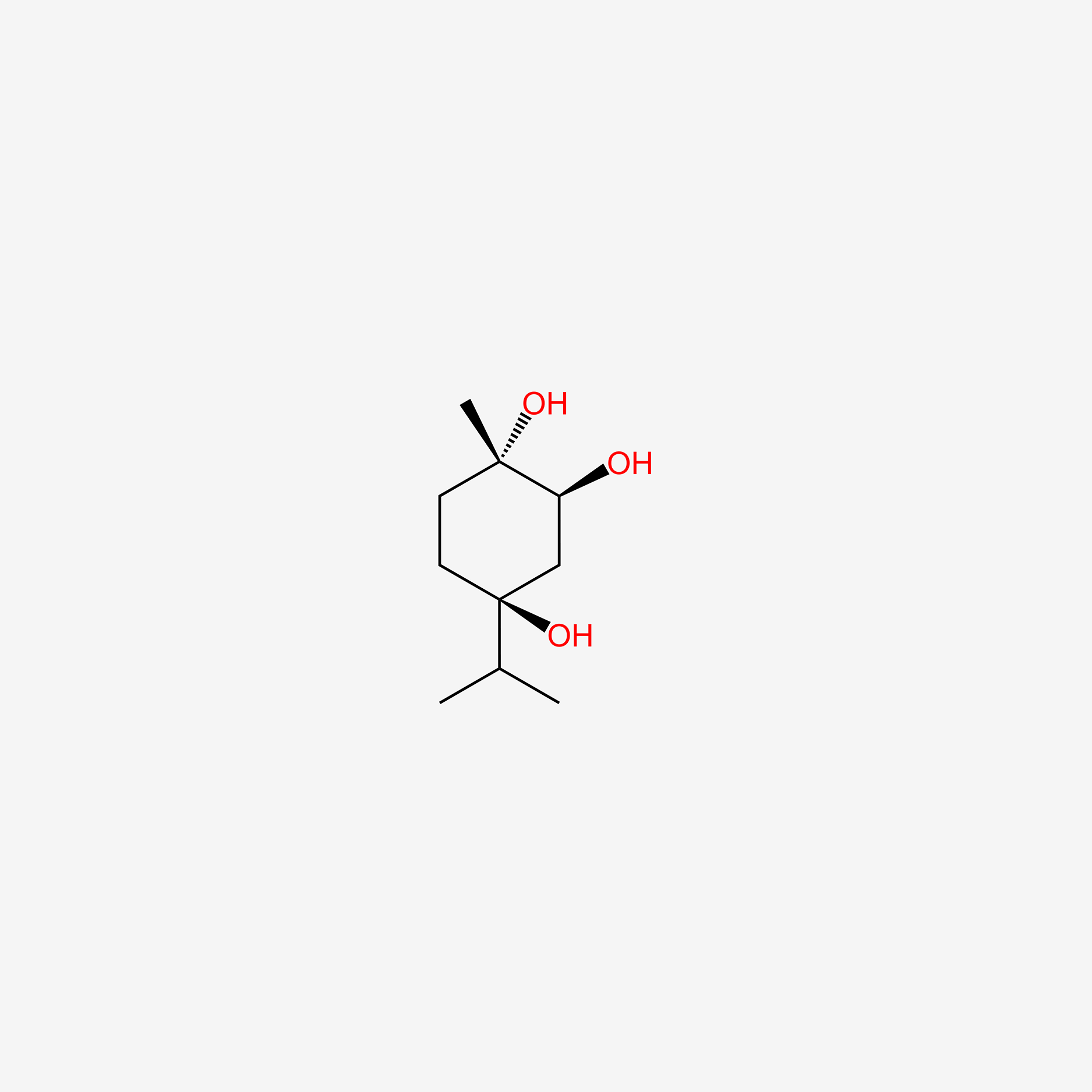 |
0.269 | D07QKN | 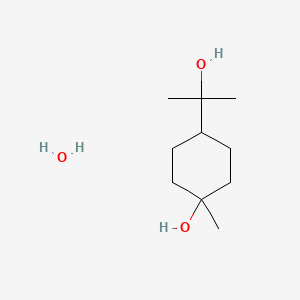 |
0.161 | ||
| ENC001145 |  |
0.250 | D0Y5ZA | 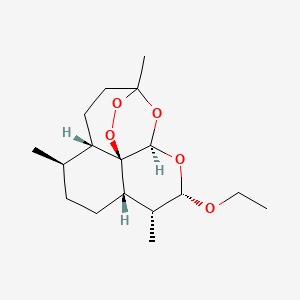 |
0.159 | ||
| ENC000388 | 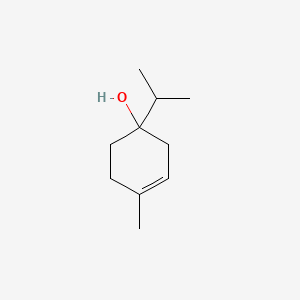 |
0.240 | D0A2AJ | 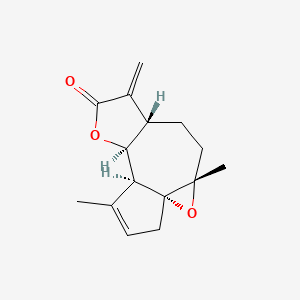 |
0.153 | ||
| ENC001637 | 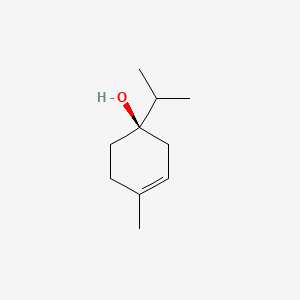 |
0.240 | D04CSZ | 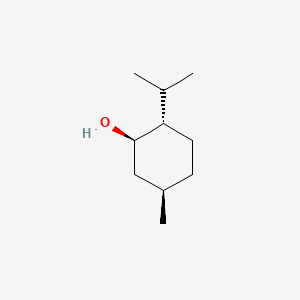 |
0.148 | ||