NPs Basic Information
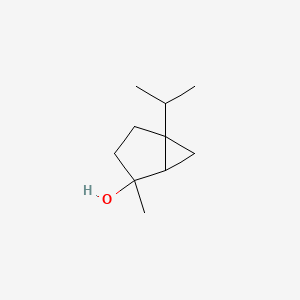
|
Name |
Sabinene hydrate
|
| Molecular Formula | C10H18O | |
| IUPAC Name* |
2-methyl-5-propan-2-ylbicyclo[3.1.0]hexan-2-ol
|
|
| SMILES |
CC(C)C12CCC(C1C2)(C)O
|
|
| InChI |
InChI=1S/C10H18O/c1-7(2)10-5-4-9(3,11)8(10)6-10/h7-8,11H,4-6H2,1-3H3
|
|
| InChIKey |
KXSDPILWMGFJMM-UHFFFAOYSA-N
|
|
| Synonyms |
Sabinene hydrate; 4-Thujanol; 546-79-2; 2-methyl-5-propan-2-ylbicyclo[3.1.0]hexan-2-ol; 5-Isopropyl-2-methylbicyclo[3.1.0]hexan-2-ol; Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-; FEMA No. 3239; cis-4-thujanol; Bicyclo(3.1.0)hexan-2-ol, 2-methyl-5-(1-methylethyl)-; cis-Sabinenhydrate; cis-Sabinene hydrate; Sabinene hydrate, cis; 4-Thujanol (natural); 4-methyl-1-propan-2-ylbicyclo[3.1.0]hexan-4-ol; cis-Sabinenehydrate; cis-Sabinene hydrate (cis for IP vs Me); EINECS 208-911-7; (Z)-Sabinene hydrate; Sabinene hydrate trans (trans for IP vs. OH); trans-Sabinene hydrate (trans for IP vs. OH); sabinene hydrate (cis-); (1R,2S,5S)-5-Isopropyl-2-methylbicyclo[3.1.0]hexan-2-ol; SCHEMBL438844; 2-Methyl-5-(1-methylethyl)bicyclo(3.1.0)hexan-2-ol; CHEBI:16377; DTXSID40862164; (1.alpha.,2.beta.,5.alpha.)-2-Methyl-5-(1-methylethyl)bicyclo(3.1.0)hexan-2-ol; 5-Isopropyl-2-methylbicyclo[3.1.0]hexan-2-ol-, (1.alpha.,2.beta.,5.alpha.)-; 4-Thujanol 100 microg/mL in Acetonitrile; DB-052630; FT-0632362; FT-0778147; Q27101876
|
|
| CAS | 546-79-2 | |
| PubChem CID | 62367 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 154.25 | ALogp: | 2.1 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 2 |
| Heavy Atoms: | 11 | QED Weighted: | 0.615 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.329 | MDCK Permeability: | 0.00002340 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.023 |
| 30% Bioavailability (F30%): | 0.224 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.778 | Plasma Protein Binding (PPB): | 71.10% |
| Volume Distribution (VD): | 1.222 | Fu: | 36.26% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.069 | CYP1A2-substrate: | 0.571 |
| CYP2C19-inhibitor: | 0.063 | CYP2C19-substrate: | 0.904 |
| CYP2C9-inhibitor: | 0.051 | CYP2C9-substrate: | 0.229 |
| CYP2D6-inhibitor: | 0.016 | CYP2D6-substrate: | 0.365 |
| CYP3A4-inhibitor: | 0.155 | CYP3A4-substrate: | 0.299 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 14.208 | Half-life (T1/2): | 0.389 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.014 | Human Hepatotoxicity (H-HT): | 0.084 |
| Drug-inuced Liver Injury (DILI): | 0.039 | AMES Toxicity: | 0.033 |
| Rat Oral Acute Toxicity: | 0.076 | Maximum Recommended Daily Dose: | 0.049 |
| Skin Sensitization: | 0.14 | Carcinogencity: | 0.123 |
| Eye Corrosion: | 0.064 | Eye Irritation: | 0.958 |
| Respiratory Toxicity: | 0.112 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002232 | 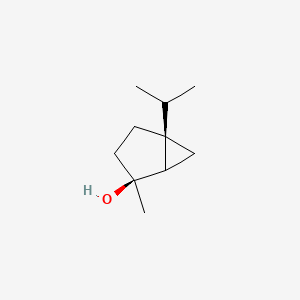 |
1.000 | D07QKN | 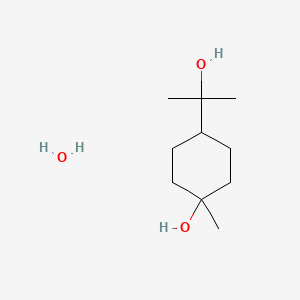 |
0.271 | ||
| ENC005252 | 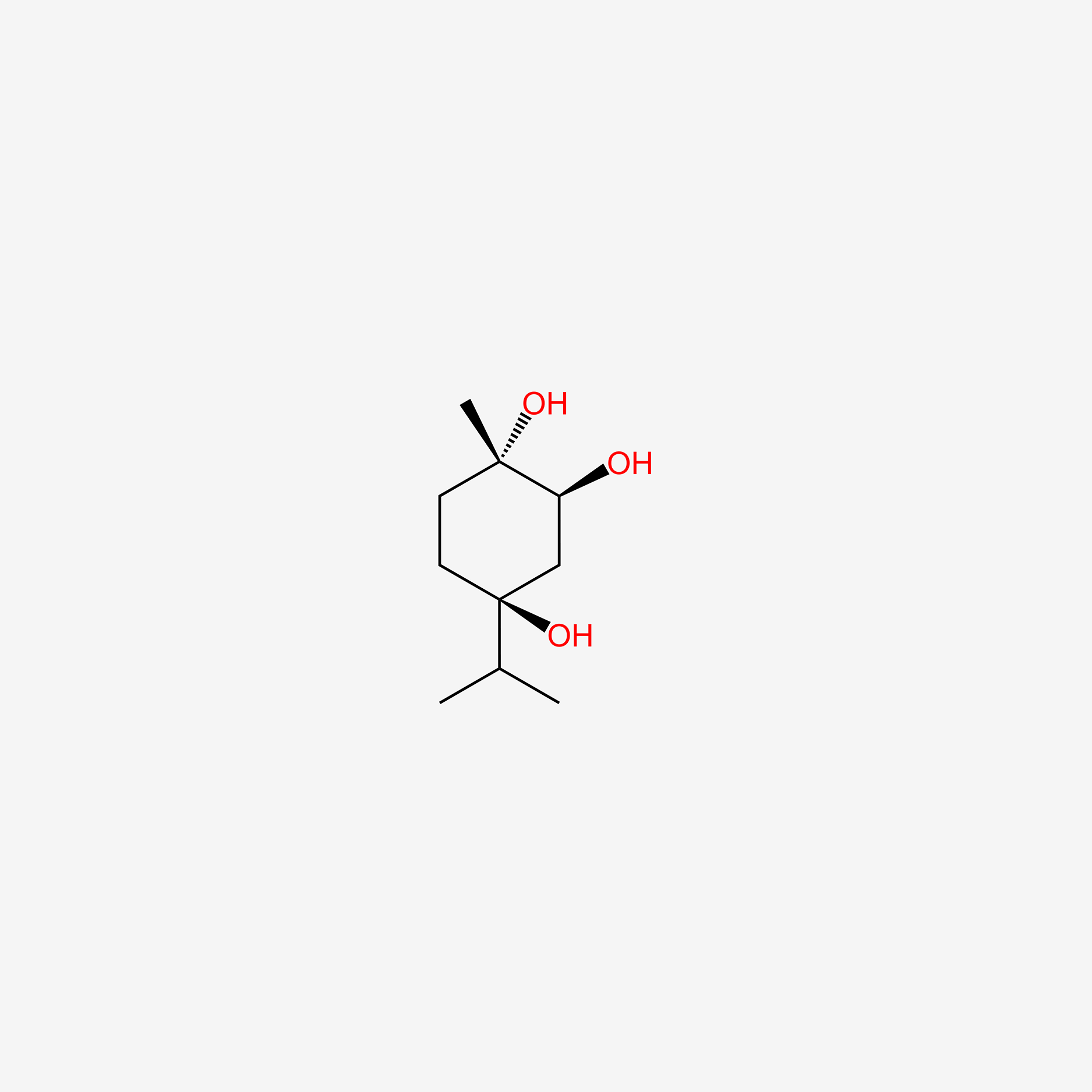 |
0.512 | D04CSZ | 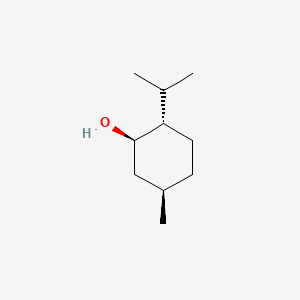 |
0.234 | ||
| ENC001814 | 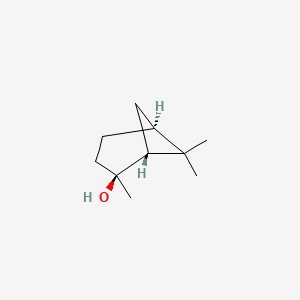 |
0.381 | D0H1QY |  |
0.234 | ||
| ENC001292 | 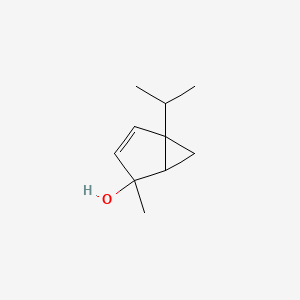 |
0.381 | D01CKY | 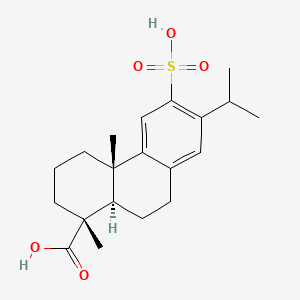 |
0.210 | ||
| ENC000528 | 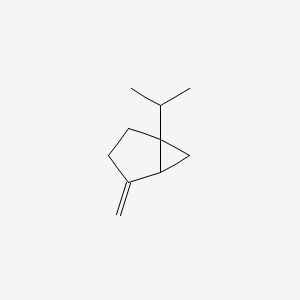 |
0.366 | D0V8HA | 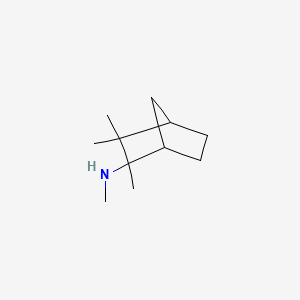 |
0.196 | ||
| ENC003100 | 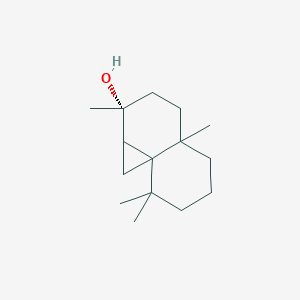 |
0.358 | D0L2LS | 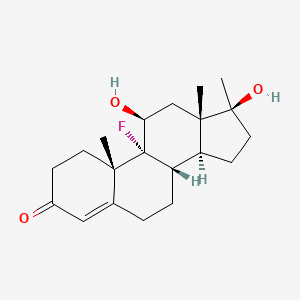 |
0.190 | ||
| ENC001893 | 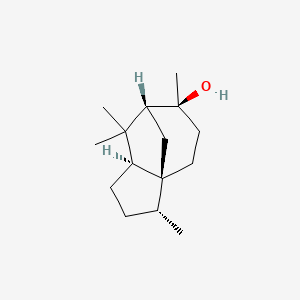 |
0.358 | D0U3GL | 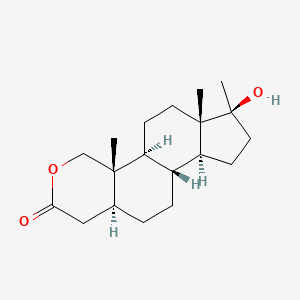 |
0.184 | ||
| ENC003098 | 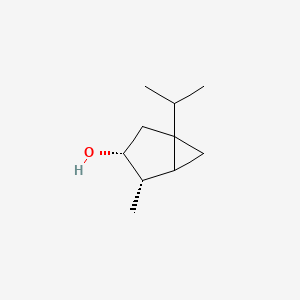 |
0.349 | D0Z1XD | 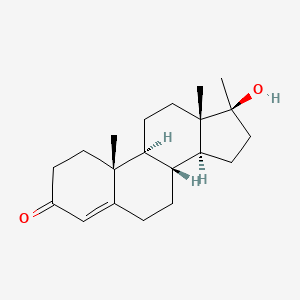 |
0.184 | ||
| ENC002220 | 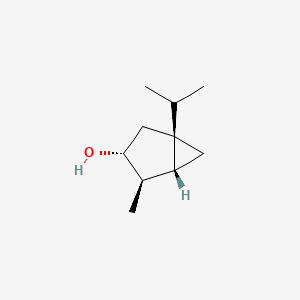 |
0.349 | D01JEU | 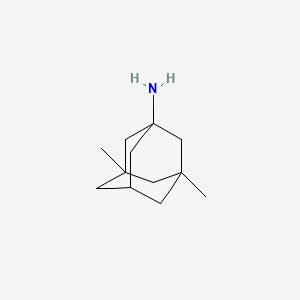 |
0.182 | ||
| ENC000331 | 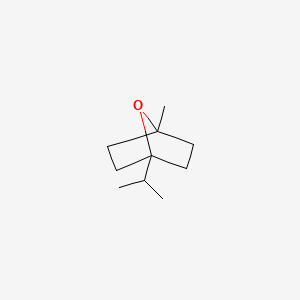 |
0.341 | D04ATM | 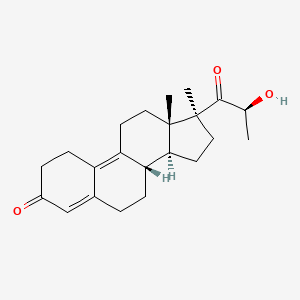 |
0.181 | ||