NPs Basic Information
|
Name |
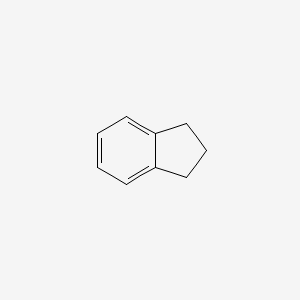
Indan
|
| Molecular Formula | C9H10 | |
| IUPAC Name* |
2,3-dihydro-1H-indene
|
|
| SMILES |
C1CC2=CC=CC=C2C1
|
|
| InChI |
InChI=1S/C9H10/c1-2-5-9-7-3-6-8(9)4-1/h1-2,4-5H,3,6-7H2
|
|
| InChIKey |
PQNFLJBBNBOBRQ-UHFFFAOYSA-N
|
|
| Synonyms |
INDAN; Indane; 2,3-Dihydro-1H-indene; 496-11-7; Hydrindene; 1H-Indene, 2,3-dihydro-; Benzocyclopentane; 2,3-Dihydroindene; 1,2-Hydrindene; Hydrindonaphthene; Indene, 2,3-dihydro-; NSC 5292; H9SCX043IG; CHEMBL370687; CHEBI:37911; NSC-5292; Indane (VAN); EINECS 207-814-7; UNII-H9SCX043IG; Dihydroindene; AI3-02275; 16N; Indene,3-dihydro-; MFCD00003795; Indan, 95%; INDAN [MI]; Indan, analytical standard; DSSTox_CID_30701; DSSTox_GSID_52132; WLN: L56T&J; DTXSID4052132; Indan 10 microg/mL in Methanol; NSC5292; STR04252; ZINC1680824; Tox21_303879; BDBM50167998; AKOS000121540; CS-W016586; NCGC00357139-01; 56573-11-6; CAS-496-11-7; DB-051655; FT-0627193; I0011; EN300-21168; D97393; A827773; Q420109; Q-201237; F0001-1290
|
|
| CAS | 496-11-7 | |
| PubChem CID | 10326 | |
| ChEMBL ID | CHEMBL370687 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 118.18 | ALogp: | 3.2 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 9 | QED Weighted: | 0.491 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.361 | MDCK Permeability: | 0.00002110 |
| Pgp-inhibitor: | 0.013 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.797 |
| 30% Bioavailability (F30%): | 0.633 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.971 | Plasma Protein Binding (PPB): | 92.67% |
| Volume Distribution (VD): | 2.604 | Fu: | 6.97% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.976 | CYP1A2-substrate: | 0.778 |
| CYP2C19-inhibitor: | 0.716 | CYP2C19-substrate: | 0.126 |
| CYP2C9-inhibitor: | 0.196 | CYP2C9-substrate: | 0.654 |
| CYP2D6-inhibitor: | 0.232 | CYP2D6-substrate: | 0.871 |
| CYP3A4-inhibitor: | 0.031 | CYP3A4-substrate: | 0.255 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.264 | Half-life (T1/2): | 0.464 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.04 | Human Hepatotoxicity (H-HT): | 0.052 |
| Drug-inuced Liver Injury (DILI): | 0.04 | AMES Toxicity: | 0.104 |
| Rat Oral Acute Toxicity: | 0.111 | Maximum Recommended Daily Dose: | 0.126 |
| Skin Sensitization: | 0.708 | Carcinogencity: | 0.468 |
| Eye Corrosion: | 0.945 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.102 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000038 | 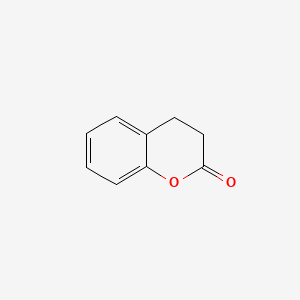 |
0.439 | D0MP5H | 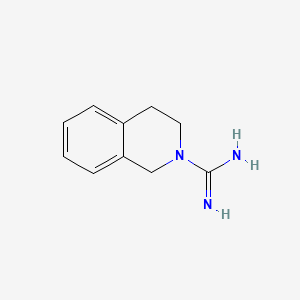 |
0.488 | ||
| ENC000518 | 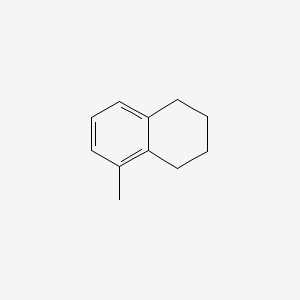 |
0.405 | D05IHU | 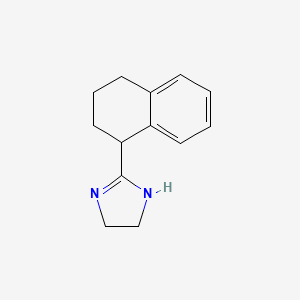 |
0.385 | ||
| ENC000673 | 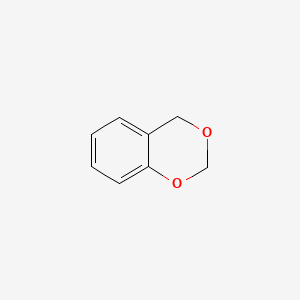 |
0.390 | D06OMW | 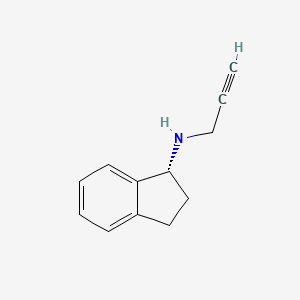 |
0.354 | ||
| ENC001319 | 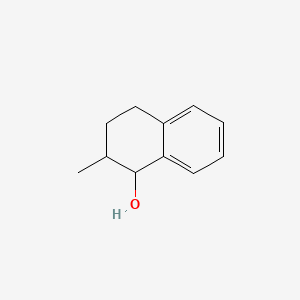 |
0.386 | D0E6YQ | 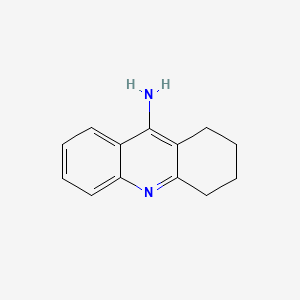 |
0.340 | ||
| ENC000681 | 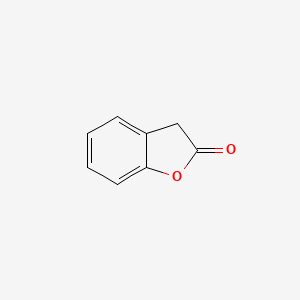 |
0.366 | D0R8PX | 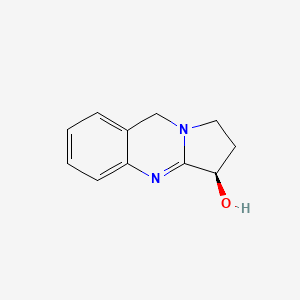 |
0.333 | ||
| ENC001380 | 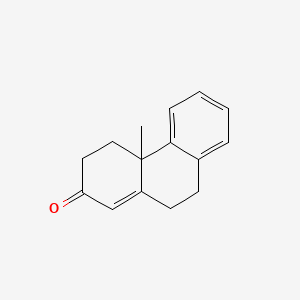 |
0.352 | D0UM7O | 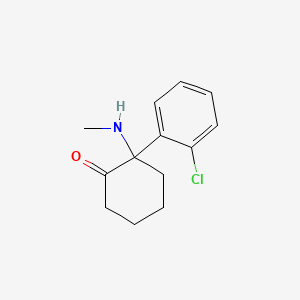 |
0.309 | ||
| ENC000159 | 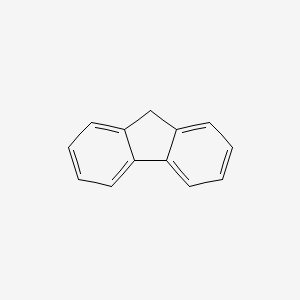 |
0.347 | D01UTL | 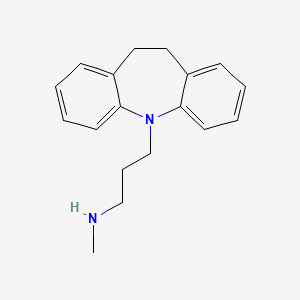 |
0.303 | ||
| ENC000171 | 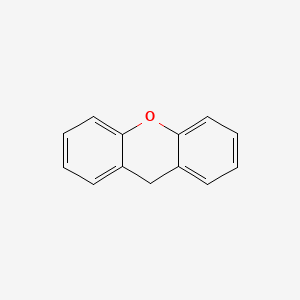 |
0.327 | D04WFD | 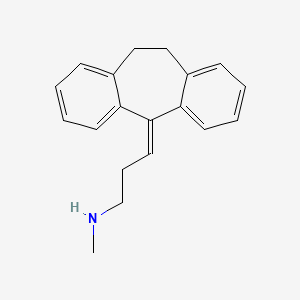 |
0.303 | ||
| ENC006142 | 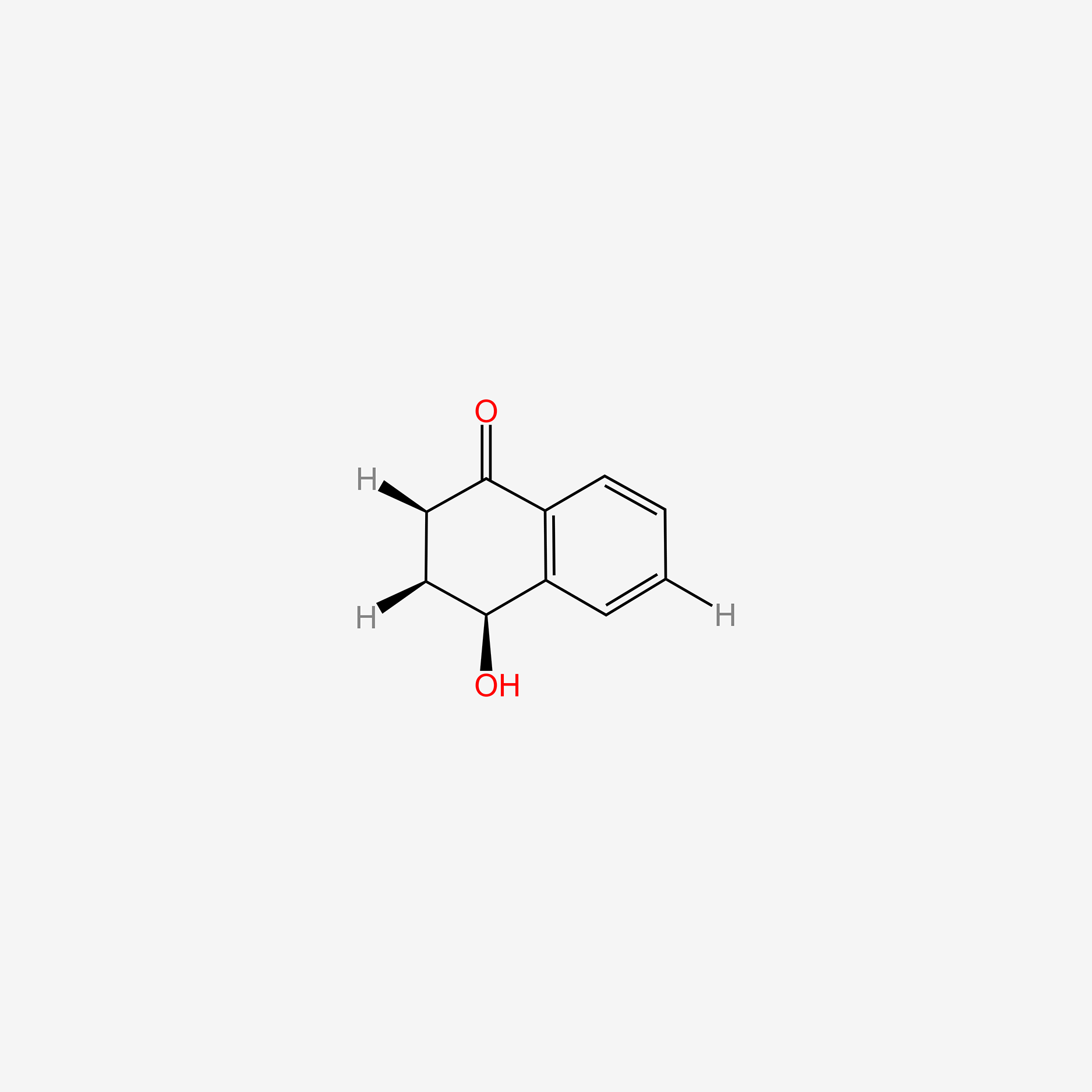 |
0.326 | D0N7AD | 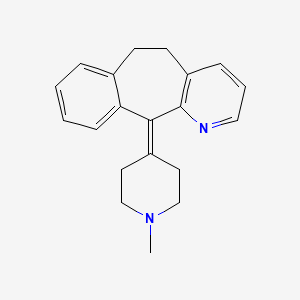 |
0.296 | ||
| ENC005244 | 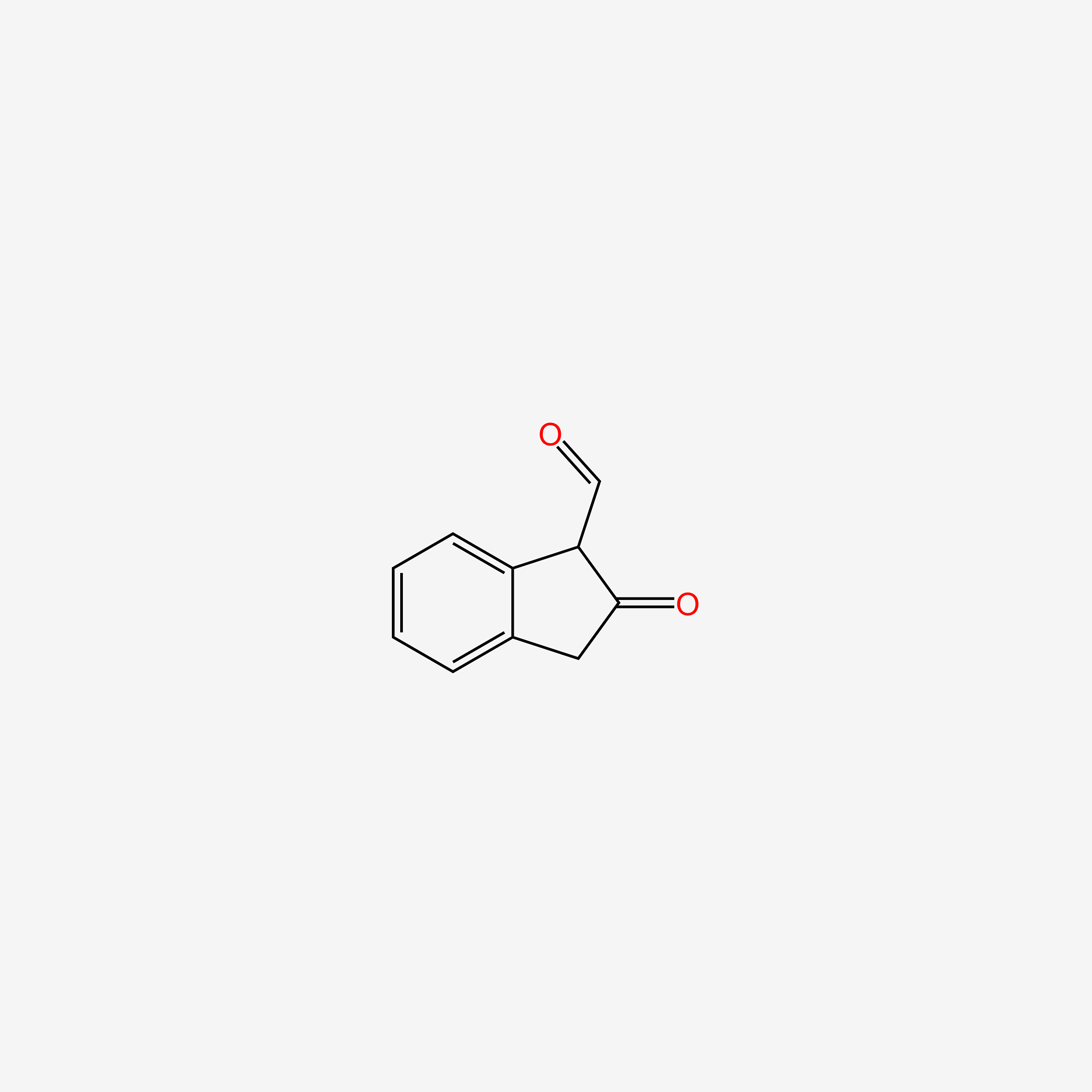 |
0.326 | D0Y5UG | 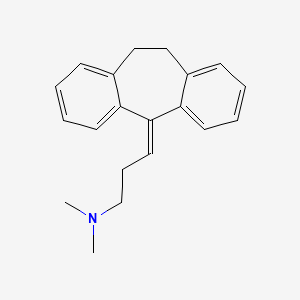 |
0.294 | ||