NPs Basic Information
|
Name |
Maltol
|
| Molecular Formula | C6H6O3 | |
| IUPAC Name* |
3-hydroxy-2-methylpyran-4-one
|
|
| SMILES |
CC1=C(C(=O)C=CO1)O
|
|
| InChI |
InChI=1S/C6H6O3/c1-4-6(8)5(7)2-3-9-4/h2-3,8H,1H3
|
|
| InChIKey |
XPCTZQVDEJYUGT-UHFFFAOYSA-N
|
|
| Synonyms |
MALTOL; 118-71-8; 3-Hydroxy-2-methyl-4-pyrone; 3-Hydroxy-2-methyl-4H-pyran-4-one; Larixinic acid; Palatone; Talmon; Larixic acid; Vetol; Veltol; Corps praline; 3-hydroxy-2-methylpyran-4-one; 4H-Pyran-4-one, 3-hydroxy-2-methyl-; 2-Methyl pyromeconic acid; 2-Methylpyromeconic acid; 2-Methyl-3-hydroxy-4-pyrone; 2-Methyl-3-hydroxypyrone; Maltol (natural); 3-Hydroxy-2-methyl-gamma-pyrone; 2-Methyl-3-oxy-gamma-pyrone; FEMA No. 2656; 3-Hydroxy-2-methylpyrone; 3-Hydroxy-2-methyl-pyran-4-one; MFCD00006578; NSC 2829; 2-methyl-3-hydroxy-4-pyranone; 3-Hydroxy-2-methyl-4-pyranone; CHEBI:69438; NSC2829; 3-Hydroxy-2-methyl-1,4-pyrone; Ins no.636; NSC-2829; 3-Hydroxy-2-methyl-.gamma.-pyrone; NSC-404458; Ins-636; MLS000069412; 3A9RD92BS4; 5-Hydroxy-6-methyl-4H-pyran-4-one; Maltol (3-Hydroxy-2-methyl-4-pyrone); E636; SMR000059093; E-636; DSSTox_CID_5523; DSSTox_RID_77818; DSSTox_GSID_25523; WLN: T6O DVJ B1 CQ; CAS-118-71-8; Maltol [NF]; CCRIS 3467; EINECS 204-271-8; BRN 0112169; UNII-3A9RD92BS4; Methylmaltol; methyl maltol; Laricinic acid; AI3-18547; NATURAL MALTOL; Spectrum_001419; Opera_ID_338; SpecPlus_000443; MALTOL [FHFI]; MALTOL [INCI]; MALTOL [FCC]; MALTOL [USP-RS]; Spectrum2_001795; Spectrum3_001351; Spectrum4_001871; Spectrum5_000462; MALTOL [II]; MALTOL [MI]; MALTOL [MART.]; bmse000538; Maltol, analytical standard; SCHEMBL4815; 3-Hydroxy-2-pyran-4-one; BSPBio_003161; KBioGR_002365; KBioSS_001899; SPECTRUM310025; 5-18-01-00114 (Beilstein Handbook Reference); MLS001424145; MLS002415738; 3-Hydroxy-2-methyl-g-pyrone; CHEMBL31422; DivK1c_006539; 3-hydroxy-2-methylpyr-4-one; SPBio_001749; QSPL 180; DTXSID0025523; 2-Methyl-3-oxy-.gamma.-pyrone; 3-hydroxy-2-methyl-4-oxopyrane; 3-hydroxyl-2-methyl-4-pyranone; FEMA 2656; HSDB 8320; KBio1_001483; KBio2_001899; KBio2_004467; KBio2_007035; KBio3_002381; XPCTZQVDEJYUGT-UHFFFAOYSA-; 3-hydroxy-2-methyl-gamma -pyrone; HMS2052K09; HMS3394K09; KUC106764N; ZINC164488; STR01642; Tox21_202215; Tox21_300118; BBL011669; BDBM50227434; CCG-38443; Maltol, natural, >=98.5%, FG; NSC404458; s4940; STK801686; 2-methyl-3-hydroxy-4H-pyran-4-one; Maltol, >=99.0%, FCC, FG; 3-Hydroxy-2-methyl-4-pyrone, 99%; AKOS005607790; 3-Hydroxy-2-Methyl-4-pyrone, natural; CS-W013504; HY-W012788; NC00350; PS-4578; SDCCGMLS-0066563.P001; 4-(a-d-Glucopyranosido)-a-glucopyranose; NCGC00091223-01; NCGC00091223-02; NCGC00091223-03; NCGC00091223-04; NCGC00091223-05; NCGC00178231-01; NCGC00254046-01; NCGC00259764-01; BP-11468; KSC-11-228-8; NCI60_002320; SY011358; DB-002512; AM20080119; FT-0615804; M0673; EN300-93557; A804081; Q420648; SR-01000712383; SR-01000712383-3; W-108539; BRD-K40619305-001-12-1; Z1255382969; Maltol, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 118-71-8 | |
| PubChem CID | 8369 | |
| ChEMBL ID | CHEMBL31422 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 126.11 | ALogp: | 0.4 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.565 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.594 | MDCK Permeability: | 0.00002430 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.033 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.261 | Plasma Protein Binding (PPB): | 83.32% |
| Volume Distribution (VD): | 0.701 | Fu: | 37.94% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.344 | CYP1A2-substrate: | 0.733 |
| CYP2C19-inhibitor: | 0.092 | CYP2C19-substrate: | 0.461 |
| CYP2C9-inhibitor: | 0.032 | CYP2C9-substrate: | 0.704 |
| CYP2D6-inhibitor: | 0.025 | CYP2D6-substrate: | 0.531 |
| CYP3A4-inhibitor: | 0.014 | CYP3A4-substrate: | 0.245 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.211 | Half-life (T1/2): | 0.848 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.054 | Human Hepatotoxicity (H-HT): | 0.116 |
| Drug-inuced Liver Injury (DILI): | 0.236 | AMES Toxicity: | 0.036 |
| Rat Oral Acute Toxicity: | 0.33 | Maximum Recommended Daily Dose: | 0.027 |
| Skin Sensitization: | 0.548 | Carcinogencity: | 0.788 |
| Eye Corrosion: | 0.545 | Eye Irritation: | 0.943 |
| Respiratory Toxicity: | 0.453 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
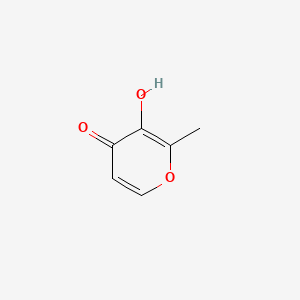
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001342 | 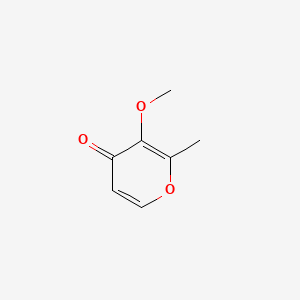 |
0.545 | D0N0OU | 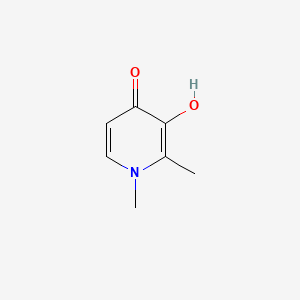 |
0.429 | ||
| ENC000746 | 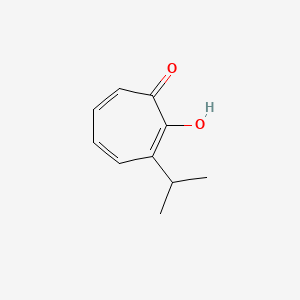 |
0.333 | D0G4KG | 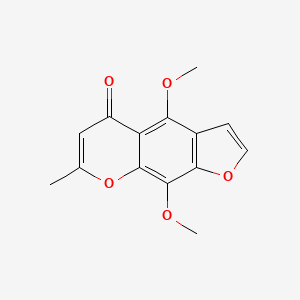 |
0.242 | ||
| ENC002801 | 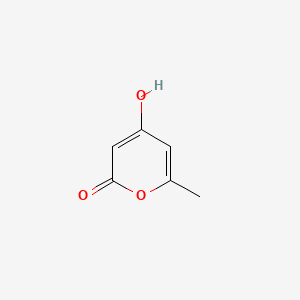 |
0.333 | D06GIP | 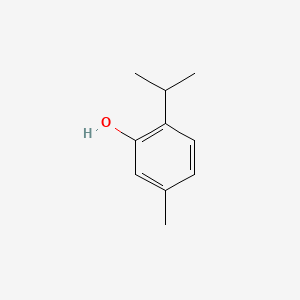 |
0.233 | ||
| ENC004823 | 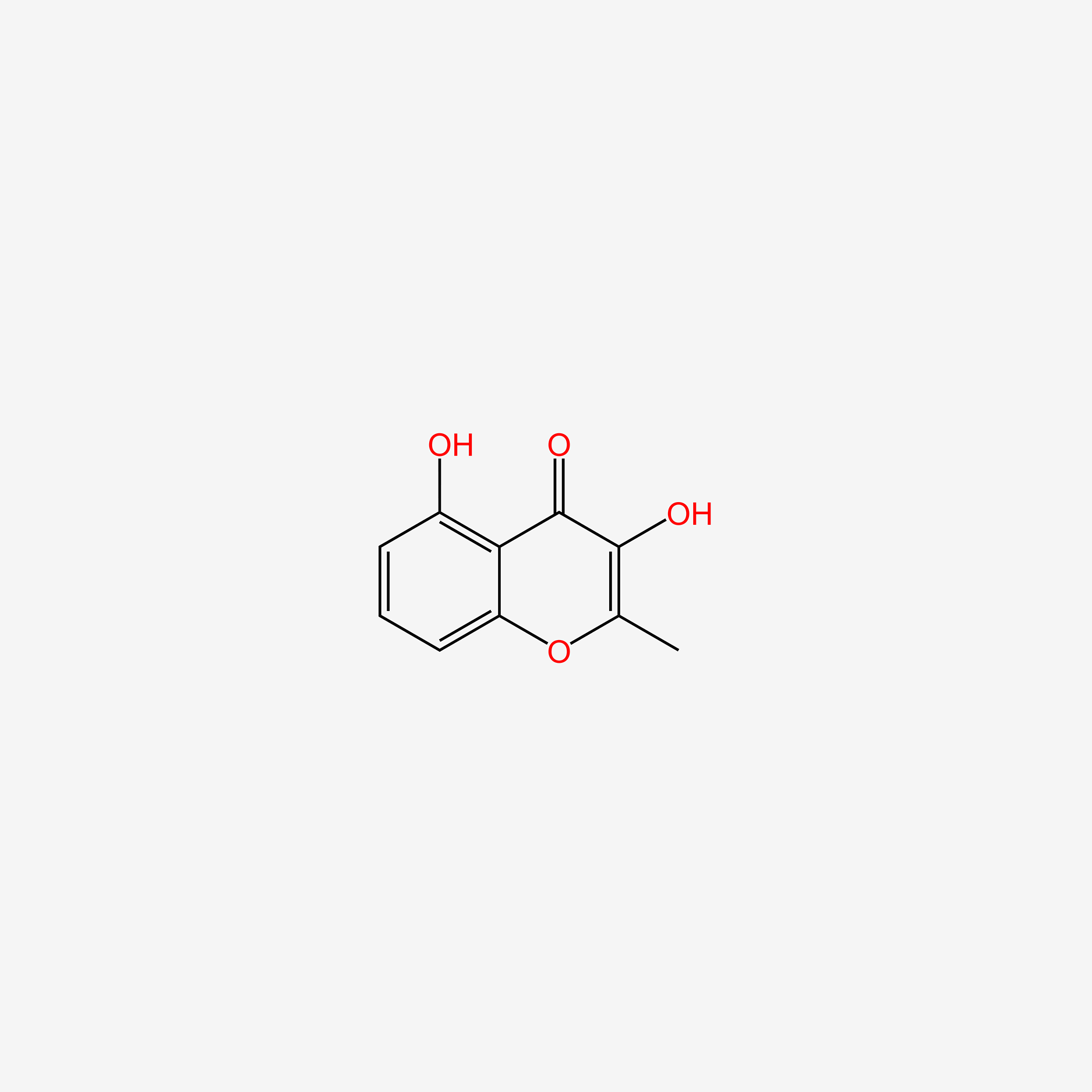 |
0.319 | D08SKH | 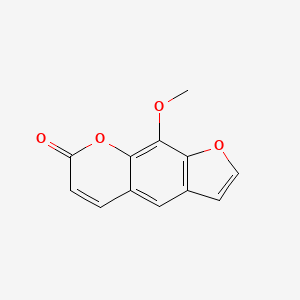 |
0.228 | ||
| ENC002874 | 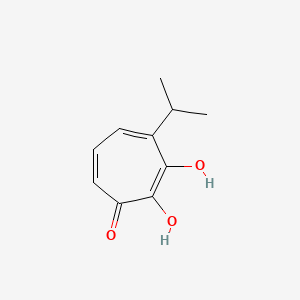 |
0.318 | D0E9CD | 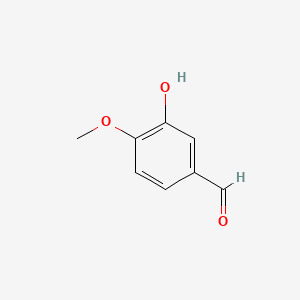 |
0.227 | ||
| ENC000101 | 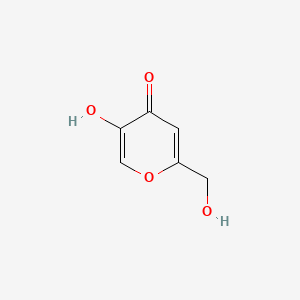 |
0.308 | D0A3HB | 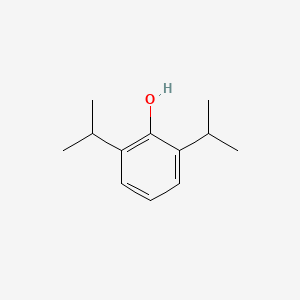 |
0.208 | ||
| ENC001447 | 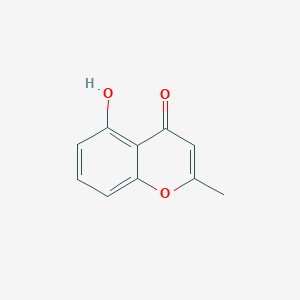 |
0.304 | D01WJL | 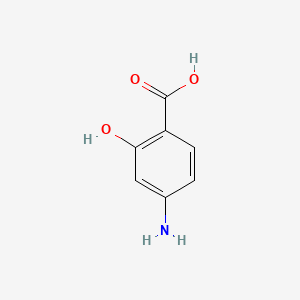 |
0.205 | ||
| ENC006095 | 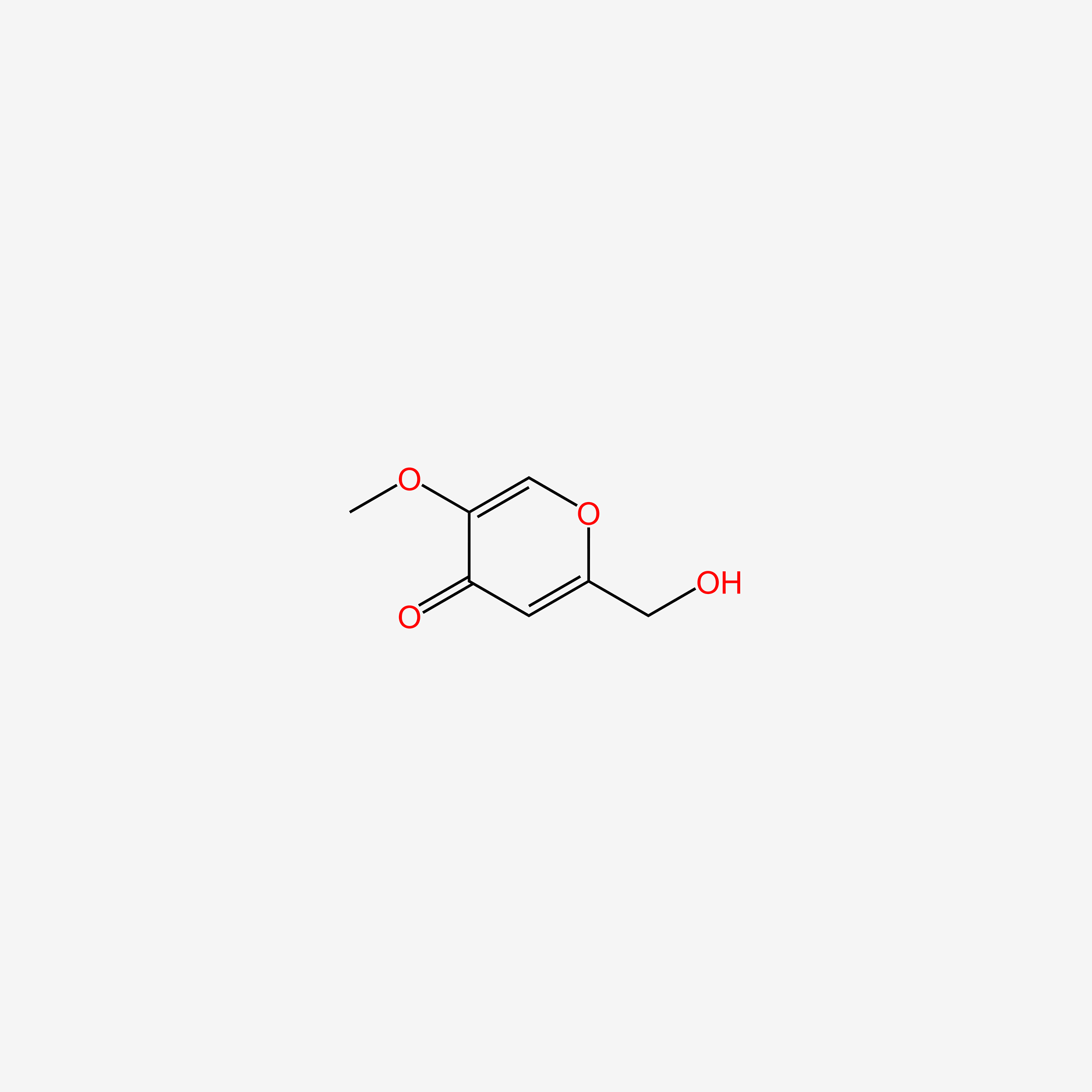 |
0.286 | D0C4YC | 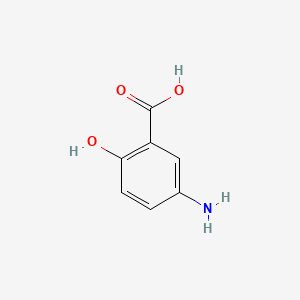 |
0.205 | ||
| ENC006096 | 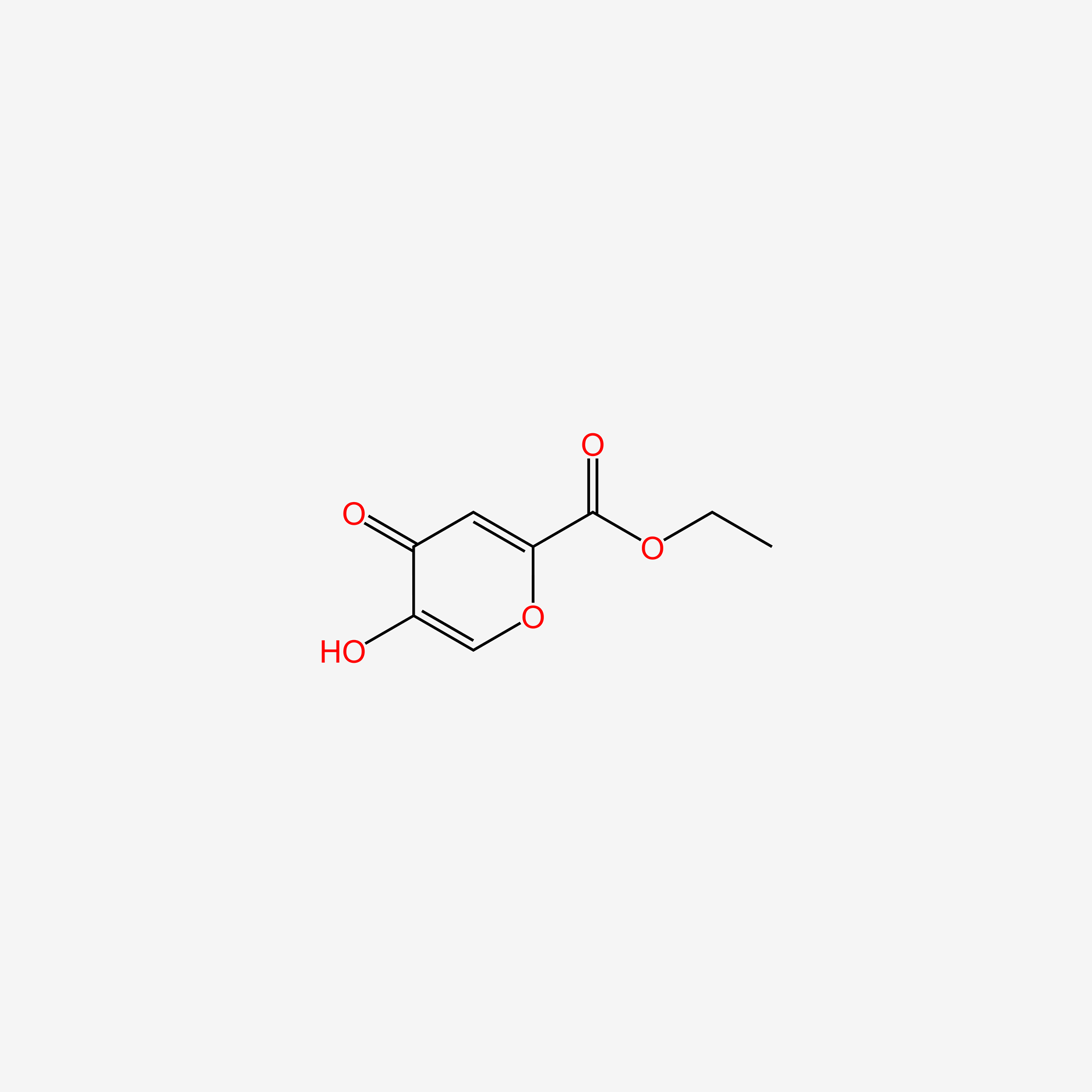 |
0.283 | D0U5QK | 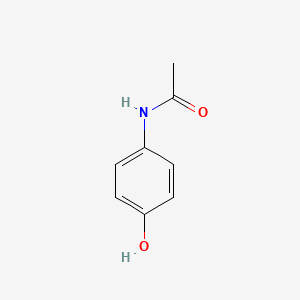 |
0.200 | ||
| ENC005125 | 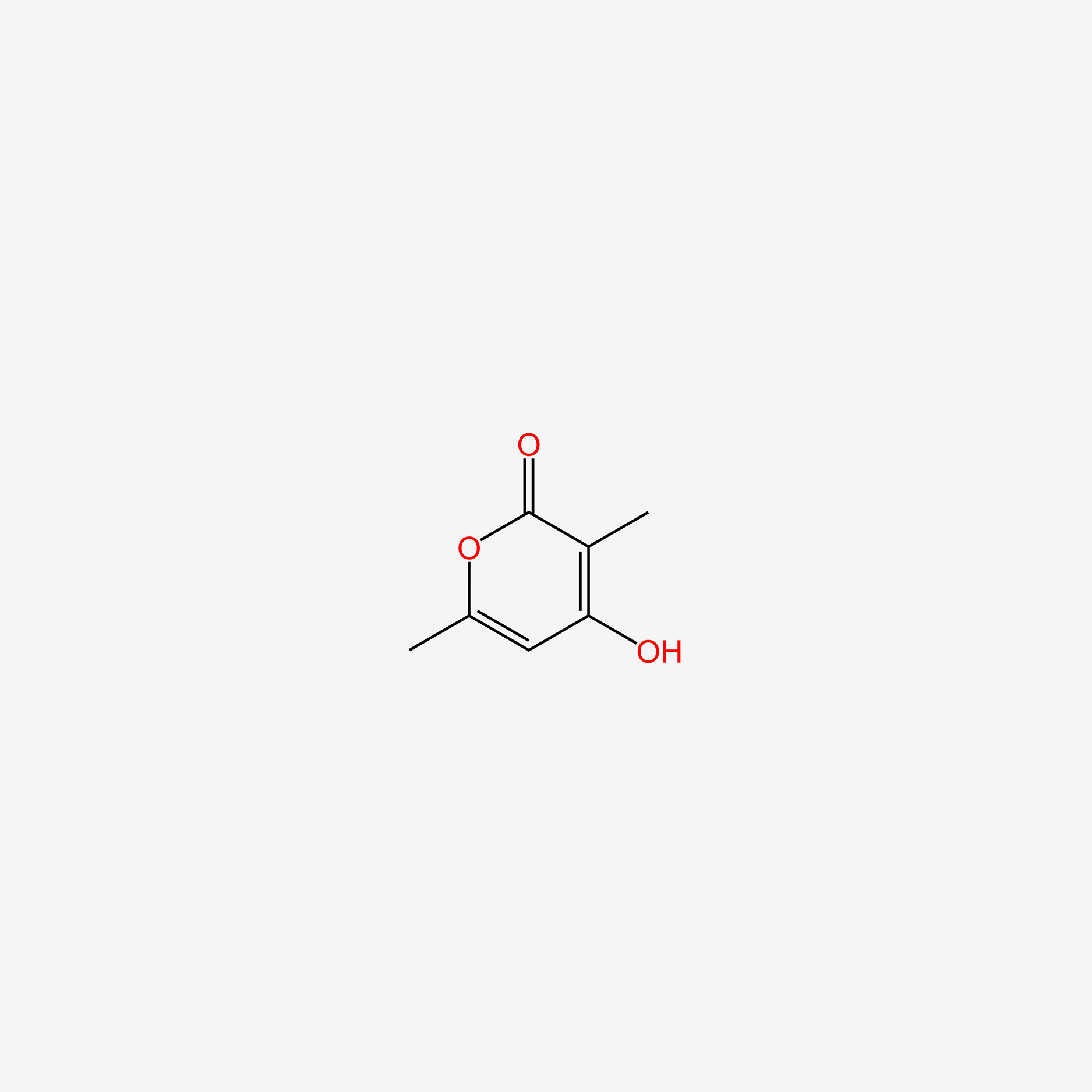 |
0.282 | D03GET | 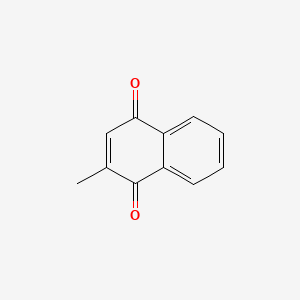 |
0.200 | ||