NPs Basic Information
|
Name |
Stearyl Alcohol
|
| Molecular Formula | C18H38O | |
| IUPAC Name* |
octadecan-1-ol
|
|
| SMILES |
CCCCCCCCCCCCCCCCCCO
|
|
| InChI |
InChI=1S/C18H38O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19/h19H,2-18H2,1H3
|
|
| InChIKey |
GLDOVTGHNKAZLK-UHFFFAOYSA-N
|
|
| Synonyms |
Stearyl alcohol; Octadecan-1-ol; 1-OCTADECANOL; Octadecanol; 112-92-5; 1-Hydroxyoctadecane; Octadecyl alcohol; n-Octadecanol; n-1-Octadecanol; Stearol; n-Octadecyl alcohol; Stearic alcohol; Atalco S; Alfol 18; Alcohol stearylicus; Steraffine; Polaax; Stenol; Crodacol-S; Aldol 62; Siponol S; Siponol SC; Lanol S; Sipol S; Adol 68; Decyl octyl alcohol; Cachalot S-43; Lorol 28; 1-0ctadecanol; Dytol E-46; Usp xiii stearyl alcohol; Stearylalkohol; Rita SA; CO-1895; Lanette 18; Hainol 18SS; Custom stearyl; Ultrapure s; Oristar sa; Octadecanol NF; Lipocol s-deo; Lipocol S; Stearyl alcohol s; Crodacol s95; Stearyl alcohol pc; Aec stearyl alcohol; Alfol 18 alcohol; Crodacol s-95; CO-1897; Nacol 18do alcohol; Nikkol stearyl alcohol; Sabonal c 18 95; Nacol 18-94 alcohol; Nacol 18-98 alcohol; Nacol 18-99 alcohol; NSC-5379; MFCD00002823; 2KR89I4H1Y; CHEBI:32154; NSC5379; 68911-61-5; NCGC00159369-02; NCGC00159369-04; Octadecylalkohol; DSSTox_CID_6935; DSSTox_RID_78262; Octadecanol, 1-; DSSTox_GSID_26935; C18 alcohol; Alcohol(C18); Rofamol; Crodacol S; 1-stearyl alcohol; CAS-112-92-5; Kalcohl 80; CCRIS 3960; Conol 30F; Kalcohl 8098; HSDB 1082; Adol 62; Conol 1675; NSC 5379; EINECS 204-017-6; Stearyl alcohol [JAN:NF]; BRN 1362907; stearylalcohol; UNII-2KR89I4H1Y; Octanodecanol; Stearal; AI3-01330; n-octadecylalcohol; Varonic BG; Crodacol S70; Crodacol S95NF; Stearyl alcohol NF; CO 1895F; EINECS 272-778-1; stearyl alcohol pure; Aec cetearyl alcohol; Cachalot S-56; Philcohol 1800; Stearyl alcohol USP; Lanette 18 DEO; Crodacol 1618; Lorol C18; OCTADECENOL-; Alfol 1618 alcohol; Alcohol cetylstearylicus; Alfol 1618e alcohol; Alfol 1618cg alcohol; 1-Octadecanol, 95%; SSD AF (Salt/Mix); EC 204-017-6; Ceteareth-20 (Salt/Mix); SCHEMBL23810; OCTADECANOL [WHO-DD]; STEARYL ALCOHOL [II]; STEARYL ALCOHOL [MI]; 4-01-00-01888 (Beilstein Handbook Reference); CHEMBL24640; Stearyl alcohol (JP17/NF); STEARYL ALCOHOL [JAN]; STEARYL ALCOHOL 98/F; STEARYL ALCOHOL 98/P; STEARYL ALCOHOL [HSDB]; STEARYL ALCOHOL [INCI]; WLN: Q18; STEARYL ALCOHOL [VANDF]; DTXSID8026935; SCHEMBL10409854; STEARYL ALCOHOL [MART.]; STEARYL ALCOHOL [USP-RS]; STEARYL ALCOHOL [WHO-DD]; CS-D1671; HY-Y1809; ZINC8214679; Tox21_111610; LMFA05000085; STL453659; 1-Octadecanol, technical grade, 80%; AKOS009031494; CACHALOT S-56 STEARYL ALCOHOL; STEARYL ALCOHOL [EP MONOGRAPH]; Tox21_111610_1; 1-Octadecanol, ReagentPlus(R), 99%; NCGC00159369-03; SY011369; 1-Octadecanol, puriss., >=99.0% (GC); FT-0761208; O0006; 1-Octadecanol, Selectophore(TM), >=99.5%; EN300-19954; 1-Octadecanol, Vetec(TM) reagent grade, 94%; D01924; A802702; L000755; Q632384; SR-01000944718; J-002873; SR-01000944718-1; Z104476204; Stearyl alcohol, European Pharmacopoeia (EP) Reference Standard; 2DEF44B7-B367-4188-89E4-531379568C74; Stearyl alcohol, United States Pharmacopeia (USP) Reference Standard; Stearyl Alcohol, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 112-92-5 | |
| PubChem CID | 8221 | |
| ChEMBL ID | CHEMBL24640 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 270.5 | ALogp: | 8.4 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 16 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 19 | QED Weighted: | 0.329 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.828 | MDCK Permeability: | 0.00001280 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.124 |
| 30% Bioavailability (F30%): | 0.995 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.059 | Plasma Protein Binding (PPB): | 97.54% |
| Volume Distribution (VD): | 3.031 | Fu: | 1.53% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.234 | CYP1A2-substrate: | 0.183 |
| CYP2C19-inhibitor: | 0.304 | CYP2C19-substrate: | 0.053 |
| CYP2C9-inhibitor: | 0.098 | CYP2C9-substrate: | 0.947 |
| CYP2D6-inhibitor: | 0.039 | CYP2D6-substrate: | 0.04 |
| CYP3A4-inhibitor: | 0.169 | CYP3A4-substrate: | 0.034 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.427 | Half-life (T1/2): | 0.103 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.27 | Human Hepatotoxicity (H-HT): | 0.01 |
| Drug-inuced Liver Injury (DILI): | 0.07 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.017 | Maximum Recommended Daily Dose: | 0.014 |
| Skin Sensitization: | 0.959 | Carcinogencity: | 0.037 |
| Eye Corrosion: | 0.994 | Eye Irritation: | 0.926 |
| Respiratory Toxicity: | 0.496 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
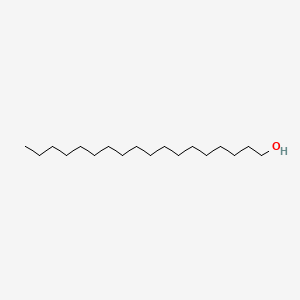
| ENC000745 | 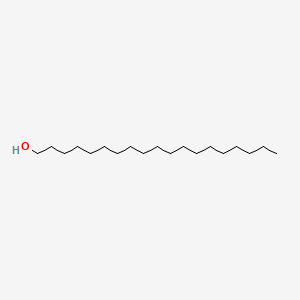 |
0.948 | D00AOJ |  |
0.821 | ||
| ENC000486 | 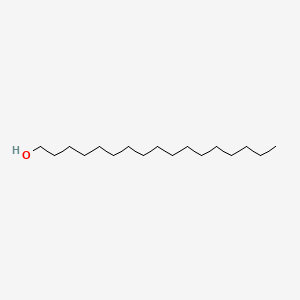 |
0.945 | D07ILQ |  |
0.667 | ||
| ENC000431 |  |
0.902 | D00FGR | 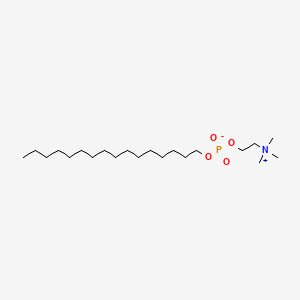 |
0.548 | ||
| ENC000082 | 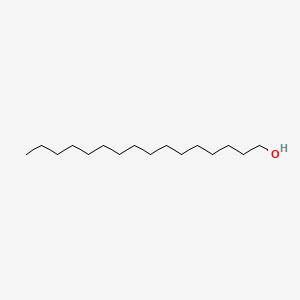 |
0.891 | D0Z5SM |  |
0.535 | ||
| ENC000761 | 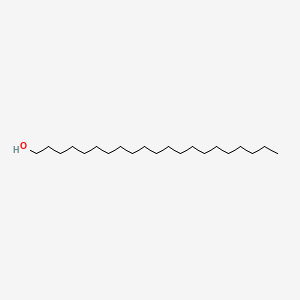 |
0.859 | D0O1PH |  |
0.494 | ||
| ENC000426 | 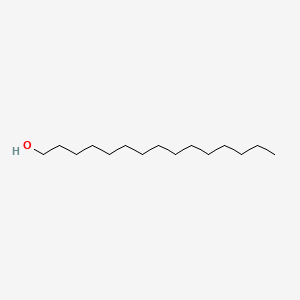 |
0.836 | D05ATI |  |
0.457 | ||
| ENC000429 |  |
0.833 | D00STJ |  |
0.444 | ||
| ENC000521 |  |
0.833 | D0P1RL |  |
0.355 | ||
| ENC000527 |  |
0.833 | D0T9TJ |  |
0.354 | ||
| ENC000428 |  |
0.833 | D0MM8N |  |
0.330 | ||