NPs Basic Information
|
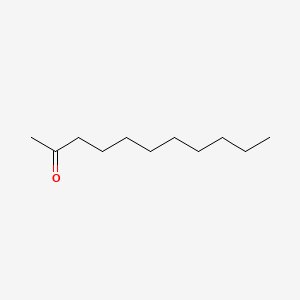
Name |
2-Undecanone
|
| Molecular Formula | C11H22O | |
| IUPAC Name* |
undecan-2-one
|
|
| SMILES |
CCCCCCCCCC(=O)C
|
|
| InChI |
InChI=1S/C11H22O/c1-3-4-5-6-7-8-9-10-11(2)12/h3-10H2,1-2H3
|
|
| InChIKey |
KYWIYKKSMDLRDC-UHFFFAOYSA-N
|
|
| Synonyms |
2-Undecanone; Undecan-2-one; Methyl nonyl ketone; 112-12-9; 2-Hendecanone; UNDECANONE; Rue ketone; Ketone, methyl nonyl; Nonyl methyl ketone; Methylnonylketone; METHYL N-NONYL KETONE; 2-Oxoundecane; MGK Dog and Cat Repellent; FEMA No. 3093; Undecanone-(2); Mgk dog & cat repellent; YV5DSO8CY9; CHEBI:17700; NSC4028; NSC-4028; 53452-70-3; Caswell No. 573O; 2-Undecanone (natural); BioUD; HSDB 7431; NSC 4028; EINECS 203-937-5; UNII-YV5DSO8CY9; MFCD00009583; EPA Pesticide Chemical Code 044102; BRN 1749573; Luparone; Enodyl; AI3-03081; methyl n-nonylketone; Methyl-n-nonylketone; MGK dog AMP MNK; 2-Undecanone, 99%; 2-Methylundecanone,(S); Methyl Nonyl Ketone FCC; UNDECANONE, 2-; DSSTox_CID_1943; MOSTIQUE EGX 101; DSSTox_RID_76418; 2-UNDECANONE [FCC]; DSSTox_GSID_21943; 2-UNDECANONE [FHFI]; 2-UNDECANONE [HSDB]; SCHEMBL117635; SCHEMBL249443; WLN: 9V1; CHEMBL1236582; DTXSID2021943; FEMA 3093; METHYL NONYL KETONE [MI]; 2-Undecanone, analytical standard; 2-Undecanone, natural, FCC, FG; ZINC1529305; Tox21_301385; BBL011441; LMFA12000002; s3762; STL146552; 2-Undecanone, >=98%, FCC, FG; AKOS005720838; CCG-266363; CS-W017685; DB08688; HY-W016969; NCGC00164003-01; NCGC00255160-01; CAS-112-12-9; VS-02950; FT-0613466; U0006; C01875; EN300-170519; F17694; A802493; Q-201393; Q2024187
|
|
| CAS | 112-12-9 | |
| PubChem CID | 8163 | |
| ChEMBL ID | CHEMBL1236582 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 170.29 | ALogp: | 4.1 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 12 | QED Weighted: | 0.493 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.512 | MDCK Permeability: | 0.00001590 |
| Pgp-inhibitor: | 0.128 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.996 |
| 30% Bioavailability (F30%): | 0.996 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.99 | Plasma Protein Binding (PPB): | 94.15% |
| Volume Distribution (VD): | 0.956 | Fu: | 5.38% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.892 | CYP1A2-substrate: | 0.685 |
| CYP2C19-inhibitor: | 0.516 | CYP2C19-substrate: | 0.482 |
| CYP2C9-inhibitor: | 0.336 | CYP2C9-substrate: | 0.936 |
| CYP2D6-inhibitor: | 0.024 | CYP2D6-substrate: | 0.374 |
| CYP3A4-inhibitor: | 0.063 | CYP3A4-substrate: | 0.106 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.928 | Half-life (T1/2): | 0.606 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.096 | Human Hepatotoxicity (H-HT): | 0.02 |
| Drug-inuced Liver Injury (DILI): | 0.122 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.036 | Maximum Recommended Daily Dose: | 0.031 |
| Skin Sensitization: | 0.768 | Carcinogencity: | 0.075 |
| Eye Corrosion: | 0.988 | Eye Irritation: | 0.963 |
| Respiratory Toxicity: | 0.328 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000556 |  |
0.917 | D0Z5BC |  |
0.500 | ||
| ENC000451 |  |
0.909 | D03ZJE |  |
0.438 | ||
| ENC000399 | 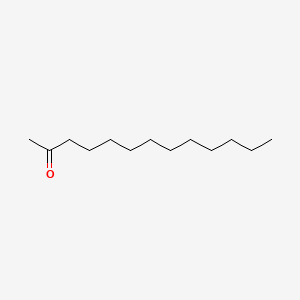 |
0.846 | D07ILQ |  |
0.431 | ||
| ENC000454 | 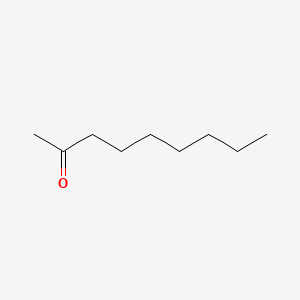 |
0.818 | D05ATI |  |
0.429 | ||
| ENC000088 | 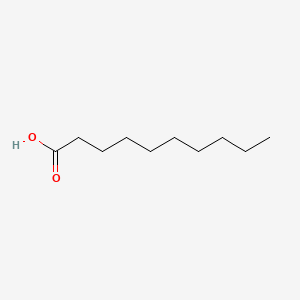 |
0.737 | D0E4WR | 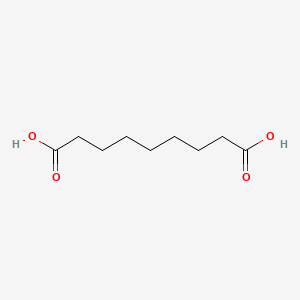 |
0.417 | ||
| ENC000722 | 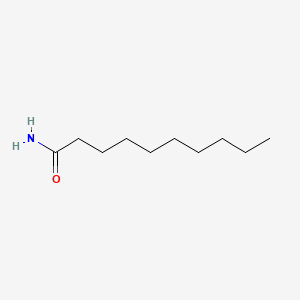 |
0.737 | D0XN8C |  |
0.415 | ||
| ENC000642 | 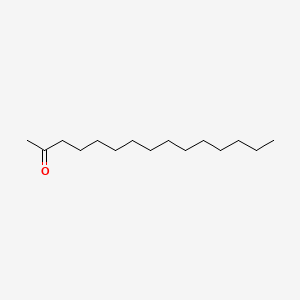 |
0.733 | D0O1PH |  |
0.394 | ||
| ENC000254 | 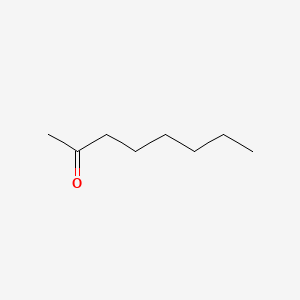 |
0.727 | D0AY9Q | 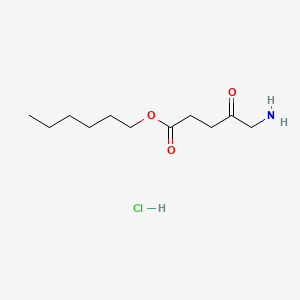 |
0.389 | ||
| ENC000249 |  |
0.725 | D0Z5SM |  |
0.381 | ||
| ENC000487 |  |
0.725 | D0G2KD |  |
0.377 | ||