NPs Basic Information
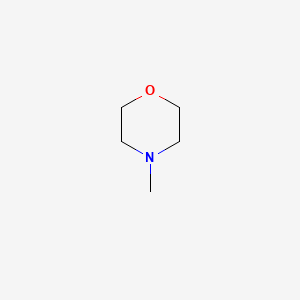
|
Name |
4-Methylmorpholine
|
| Molecular Formula | C5H11NO | |
| IUPAC Name* |
4-methylmorpholine
|
|
| SMILES |
CN1CCOCC1
|
|
| InChI |
InChI=1S/C5H11NO/c1-6-2-4-7-5-3-6/h2-5H2,1H3
|
|
| InChIKey |
SJRJJKPEHAURKC-UHFFFAOYSA-N
|
|
| Synonyms |
4-Methylmorpholine; N-METHYLMORPHOLINE; 109-02-4; Morpholine, 4-methyl-; Methylmorpholine; 1-Methylmorpholine; 4-methylmorpholin; Morpholine, N-methyl-; N-methyl morpholine; 4-Methylmorfolin; NSC 9382; 4-methyl-morpholine; 11P91ANU5X; NMM; NSC-9382; DSSTox_CID_9146; DSSTox_RID_78682; DSSTox_GSID_29146; 4-Methylmorfolin [Czech]; N-Methylmorpholin; N-methyl morpholine hydrochloride; CAS-109-02-4; CCRIS 6691; EINECS 203-640-0; UN2535; UNII-11P91ANU5X; AI3-24289; 4methylmorpholine; methyl-morpholine; N-methylmopholine; N-methylmorphline; N-methymorpholine; N-metylmorpholine; 4-methlmorpholine; 4-methylmopholine; 4-methymorpholine; N-methyhnorpholine; N-methyimorpholine; Texacat NMM; 4-methyimorpholine; 4-Methyl-1-oxa-4-azacyclohexane; N-methyl morpholin; N-methyl-mopholine; N-methyl-morpholin; N-metyl-morpholine; 4-methyl morpholin; N -methylmorpholine; N- methylmorpholine; N--methylmorpholine; N-mehtyl morpholine; N-methylmor-pholine; N-methylmorpho-line; p-Methyl morpholine; 4 -methylmorpholine; 4-methyl morpholine; MFCD00006175; 4-N-methylmorpholine; N- methyl morpholine; N-methyl -morpholine; 4-(methyl)morpholine; morpholine, 4-methyl; EC 203-640-0; SCHEMBL4622; WLN: T6N DOTJ A1; 4-Methylmorpholine, redistilled; N-METHYLMORPHOLINE [MI]; CHEMBL2448839; DTXSID9029146; NSC9382; BCP31356; STR02354; Tox21_202412; Tox21_303402; STL294217; ZINC19230118; AKOS000118797; UN 2535; 4-Methylmorpholine or n-methylmorpholine; NCGC00249222-01; NCGC00257448-01; NCGC00259961-01; BP-20398; 4-Methylmorpholine, ReagentPlus(R), 99%; DB-059805; FT-0648820; FT-0658371; FT-0701320; M0370; 4-Methylmorpholine, purum, >=98.0% (GC); EN300-18961; N-Methyl morphofine pound>>Morpholine, 4-methyl-; N-Methylmorpholine, SAJ special grade, >=99.0%; J-002223; J-515790; Q2542075; F0001-0190; 4-Methylmorpholine, purified by redistillation, >=99.5%; 4-Methylmorpholine or n-methylmorpholine [UN2535] [Flammable liquid]; 4-Methylmorpholine, BioXtra, suitable for protein sequencing, >=99.5% (GC)
|
|
| CAS | 109-02-4 | |
| PubChem CID | 7972 | |
| ChEMBL ID | CHEMBL2448839 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 101.15 | ALogp: | -0.3 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 12.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 7 | QED Weighted: | 0.437 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.388 | MDCK Permeability: | 0.00001010 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.292 |
| 30% Bioavailability (F30%): | 0.011 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.962 | Plasma Protein Binding (PPB): | 9.79% |
| Volume Distribution (VD): | 1.178 | Fu: | 89.97% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.031 | CYP1A2-substrate: | 0.216 |
| CYP2C19-inhibitor: | 0.023 | CYP2C19-substrate: | 0.934 |
| CYP2C9-inhibitor: | 0.002 | CYP2C9-substrate: | 0.259 |
| CYP2D6-inhibitor: | 0.004 | CYP2D6-substrate: | 0.87 |
| CYP3A4-inhibitor: | 0.003 | CYP3A4-substrate: | 0.202 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.833 | Half-life (T1/2): | 0.512 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.031 | Human Hepatotoxicity (H-HT): | 0.07 |
| Drug-inuced Liver Injury (DILI): | 0.028 | AMES Toxicity: | 0.075 |
| Rat Oral Acute Toxicity: | 0.403 | Maximum Recommended Daily Dose: | 0.016 |
| Skin Sensitization: | 0.836 | Carcinogencity: | 0.893 |
| Eye Corrosion: | 0.969 | Eye Irritation: | 0.934 |
| Respiratory Toxicity: | 0.829 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000895 | 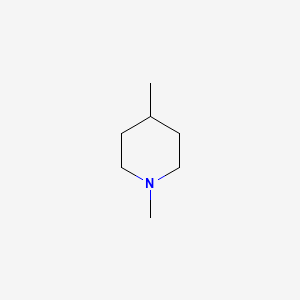 |
0.355 | D09TPF | 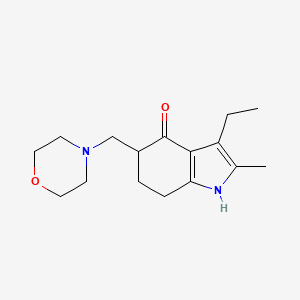 |
0.242 | ||
| ENC001185 | 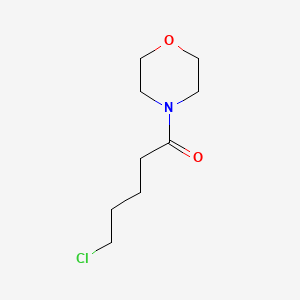 |
0.326 | D01ZSO | 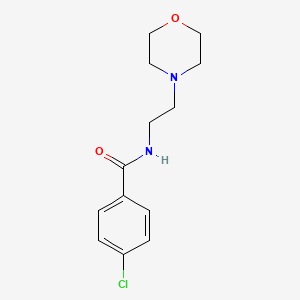 |
0.241 | ||
| ENC000615 | 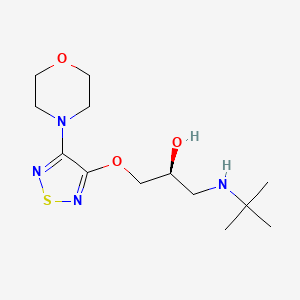 |
0.234 | D05UVD | 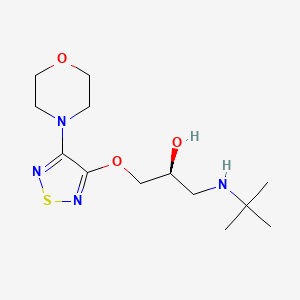 |
0.234 | ||
| ENC000579 | 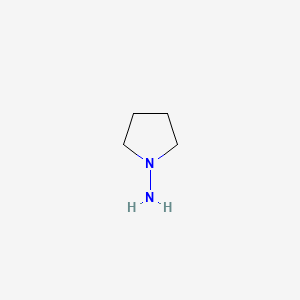 |
0.233 | D06RCB | 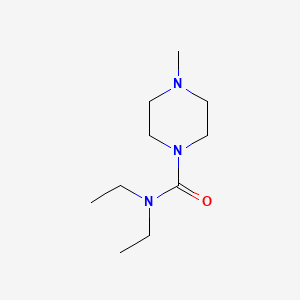 |
0.234 | ||
| ENC000751 | 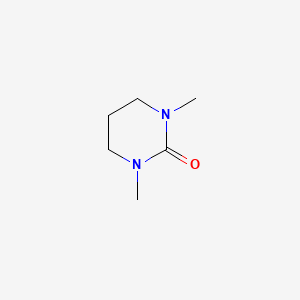 |
0.222 | D0V4UF | 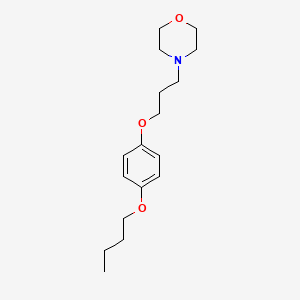 |
0.224 | ||
| ENC001384 | 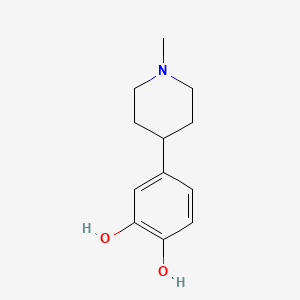 |
0.216 | D0W8SJ | 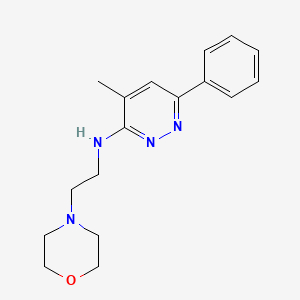 |
0.214 | ||
| ENC004066 | 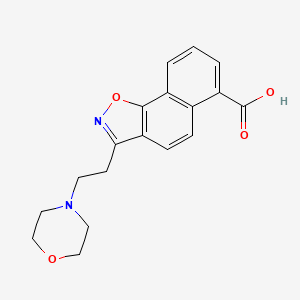 |
0.184 | D07UYO | 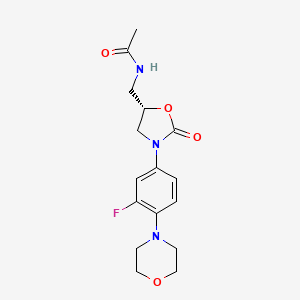 |
0.205 | ||
| ENC000817 | 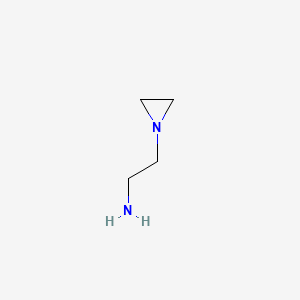 |
0.161 | D00UYE | 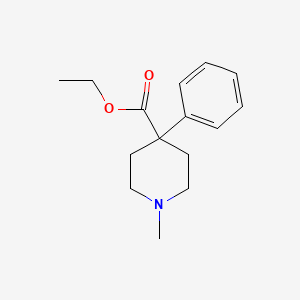 |
0.183 | ||
| ENC001488 | 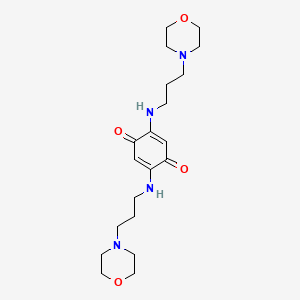 |
0.159 | D0M7BT | 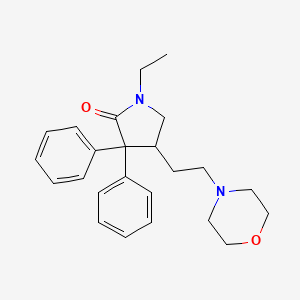 |
0.172 | ||
| ENC004863 | 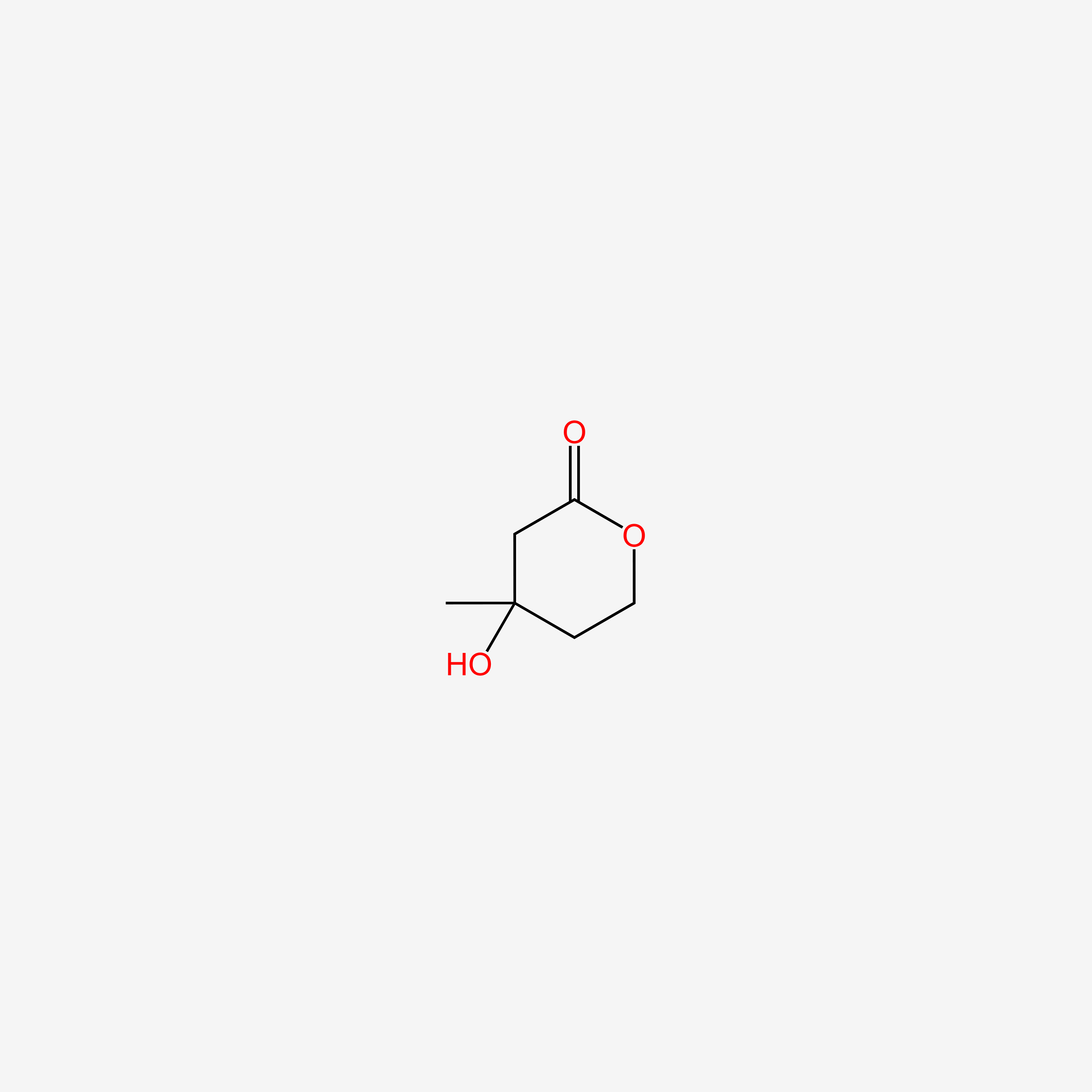 |
0.158 | D05AFX | 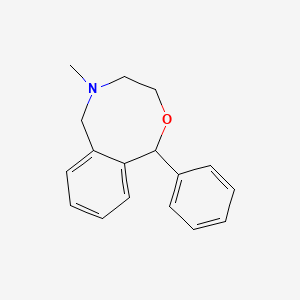 |
0.169 | ||