NPs Basic Information
|
Name |
Carvone
|
| Molecular Formula | C10H14O | |
| IUPAC Name* |
2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one
|
|
| SMILES |
CC1=CCC(CC1=O)C(=C)C
|
|
| InChI |
InChI=1S/C10H14O/c1-7(2)9-5-4-8(3)10(11)6-9/h4,9H,1,5-6H2,2-3H3
|
|
| InChIKey |
ULDHMXUKGWMISQ-UHFFFAOYSA-N
|
|
| Synonyms |
CARVONE; 99-49-0; 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-enone; Karvon; dl-Carvone; 1-Carvone; p-Mentha-6,8-dien-2-one; 2-Methyl-5-isopropenyl-2-cyclohexenone; Carvone [ISO]; D-Cavone; 2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one; 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-; 6,8(9)-p-Menthadien-2-one; 2-Methyl-5-(1-methylethenyl)-2-cyclohexen-1-one; NCI-C55867; 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one; (+/-)-carvone; 6,8-p-Menthadien-2-one; 75GK9XIA8I; p-Mentha-6,8-dien-2-one, (R)-(-)-; 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-, (R)-; CHEBI:38265; p-mentha-1(6),8-dien-2-one; NSC6275; NSC-6275; 2-Methyl-5-(1-methylethenyl)-2-cyclohexene-1-one; MFCD00062996; Carvone 100 microg/mL in Acetonitrile; Carvone (natural); l-6,8(9)-p-Menthadien-2-one; FEMA Number 2249; 6,8-p-Menthadien-2-on; limonen-6-one; d-p-Mentha-1(6),8-dien-2-one; FEMA No. 2249; HSDB 707; (+-)-Carvone; NSC 6275; EINECS 202-759-5; UNII-75GK9XIA8I; delta(sup 6,8)-(9)-terpadienone-2; BRN 1364206; Carvon; a carvone; AI3-08877; MFCD00001578; MFCD00062997; d-p-Mentha-6,8,(9)-dien-2-one; .alpha.-Carvone; delta-1-Methyl-4-isopropenyl-6-cyclohexen-2-one; 5-isopropenyl-2-methylcyclohex-2-en-1-one; CARVONE [HSDB]; CARVONE [INCI]; CARVONE [MI]; CARVONE, DL-; 5-isopropenyl-2-methyl-cyclohex-2-en-1-one; 2-Methyl-5-(1-propen-2-yl)-2-cyclohexenone; DSSTox_CID_27426; DSSTox_RID_82339; NCIOpen2_001348; (RS)-5-isopropenyl-2-methylcyclohex-2-en-1-one; DSSTox_GSID_47426; SCHEMBL39408; 4-07-00-00316 (Beilstein Handbook Reference); CARVONE DL-FORM [MI]; CHEMBL15676; CARVONE, (+-)-; CARVONE, (+/-)-; DTXSID8047426; AMY4152; HMS1789N08; NSC93738; Tox21_302547; BBL010103; NSC-93738; STK801456; AKOS000121377; AKOS016843655; CAS-99-49-0; NCGC00256915-01; WLN: L6V BUTJ B1 EY1 & U1; .delta.(sup 6,8)-(9)-Terpadienone-2; AS-10471; NCI60_008753; SY010704; SY012922; SY274718; 2-Methyl-5-isopropenyl-2-cyclohexen-1-one; DB-054736; CS-0033814; FT-0600385; FT-0605067; FT-0658046; EN300-16634; FEMA NO. 2249, (+/-)-; O10834; (-)-2-Methyl-5-isopropenyl-2-cyclohexen-1-one; A858458; Q416800; .delta.-1-Methyl-4-isopropenyl-6-cyclohexen-2-one; 5-Isopropenyl-2-methyl-2-cyclohexen-1-one, (R)-; W-100036; 2-methyl-5-(1-methyl-1-ethenyl)-2-cyclohexen-1-one
|
|
| CAS | 99-49-0 | |
| PubChem CID | 7439 | |
| ChEMBL ID | CHEMBL15676 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 150.22 | ALogp: | 2.4 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.524 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.366 | MDCK Permeability: | 0.00002790 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.02 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.99 | Plasma Protein Binding (PPB): | 52.96% |
| Volume Distribution (VD): | 0.84 | Fu: | 39.35% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.126 | CYP1A2-substrate: | 0.816 |
| CYP2C19-inhibitor: | 0.051 | CYP2C19-substrate: | 0.824 |
| CYP2C9-inhibitor: | 0.022 | CYP2C9-substrate: | 0.211 |
| CYP2D6-inhibitor: | 0.013 | CYP2D6-substrate: | 0.734 |
| CYP3A4-inhibitor: | 0.1 | CYP3A4-substrate: | 0.291 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.606 | Half-life (T1/2): | 0.766 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.005 | Human Hepatotoxicity (H-HT): | 0.046 |
| Drug-inuced Liver Injury (DILI): | 0.455 | AMES Toxicity: | 0.029 |
| Rat Oral Acute Toxicity: | 0.061 | Maximum Recommended Daily Dose: | 0.032 |
| Skin Sensitization: | 0.041 | Carcinogencity: | 0.432 |
| Eye Corrosion: | 0.832 | Eye Irritation: | 0.912 |
| Respiratory Toxicity: | 0.694 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
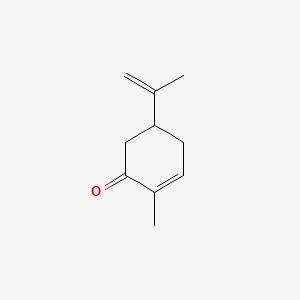
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001837 | 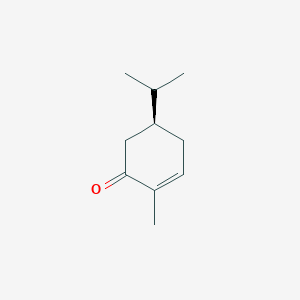 |
0.487 | D0Z8SF |  |
0.217 | ||
| ENC001066 |  |
0.474 | D0A2AJ | 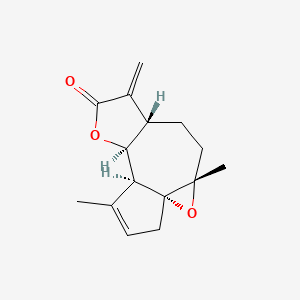 |
0.197 | ||
| ENC000555 | 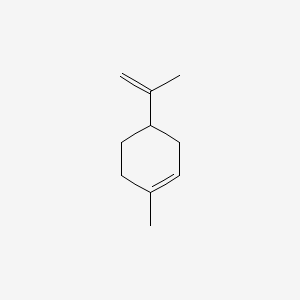 |
0.474 | D0H1QY |  |
0.184 | ||
| ENC002219 | 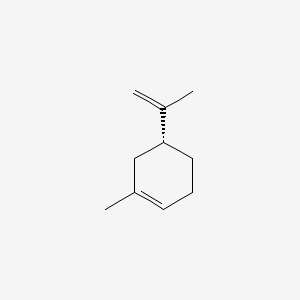 |
0.436 | D0H6VY |  |
0.182 | ||
| ENC002276 | 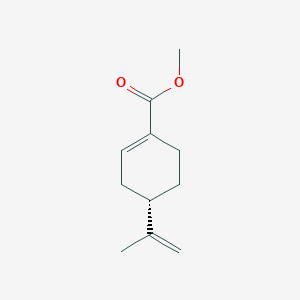 |
0.391 | D0O1UZ |  |
0.179 | ||
| ENC000567 |  |
0.381 | D06XWB |  |
0.179 | ||
| ENC001439 |  |
0.380 | D0K0EK |  |
0.173 | ||
| ENC000369 | 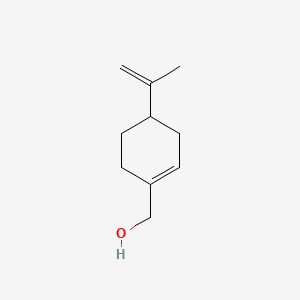 |
0.372 | D0N0OU | 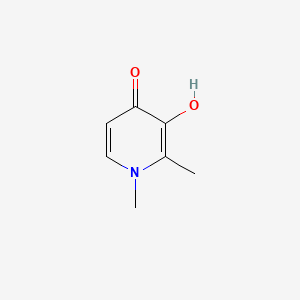 |
0.170 | ||
| ENC002100 | 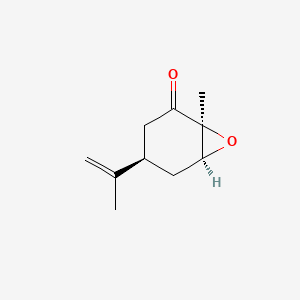 |
0.356 | D0CT4D | 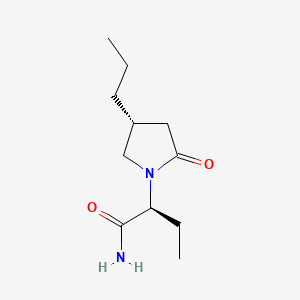 |
0.169 | ||
| ENC001829 | 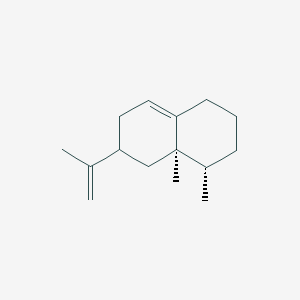 |
0.321 | D04GJN | 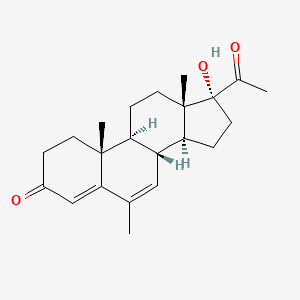 |
0.169 | ||