NPs Basic Information
|
Name |
Bromhexine
|
| Molecular Formula | C14H20Br2N2 | |
| IUPAC Name* |
2,4-dibromo-6-[[cyclohexyl(methyl)amino]methyl]aniline
|
|
| SMILES |
CN(CC1=C(C(=CC(=C1)Br)Br)N)C2CCCCC2
|
|
| InChI |
InChI=1S/C14H20Br2N2/c1-18(12-5-3-2-4-6-12)9-10-7-11(15)8-13(16)14(10)17/h7-8,12H,2-6,9,17H2,1H3
|
|
| InChIKey |
OJGDCBLYJGHCIH-UHFFFAOYSA-N
|
|
| Synonyms |
bromhexine; 3572-43-8; Benzenemethanamine, 2-amino-3,5-dibromo-N-cyclohexyl-N-methyl-; Bromhexinum; Bromhexina; Fluibron; 2,4-Dibromo-6-((cyclohexyl(methyl)amino)methyl)aniline; 2,4-dibromo-6-[[cyclohexyl(methyl)amino]methyl]aniline; 2,4-dibromo-6-{[cyclohexyl(methyl)amino]methyl}aniline; Q1J152VB1P; CHEBI:77032; Bromhexine (INN); BROMHEXINE [INN]; Bromhexine [INN:BAN]; Toluene-.alpha.,2-diamine, 3,5-dibromo-N.alpha.-cyclohexyl-N.alpha.-methyl-; N-cyclohexyl-N-methyl-(2-amino-3,5-dibrombenzyl)amine; N-2-AMINO-3,5-DIBROMOBENZYL-N-CYCLOHEXYLMETHYLAMINE; Bromexine; Bromhexinum [INN-Latin]; 3,5-dibromo-N(alpha)-cyclohexyl-N(alpha)-methyltoluene-alpha-2-diamine; Bromhexina [INN-Spanish]; SMR001826324; EINECS 222-684-1; UNII-Q1J152VB1P; NA274; Fluibron (TN); N-Cyclohexyl-N-methyl-(2-amino-3,5-dibrombenzyl)amin; Spectrum_001392; BROMHEXINE [MI]; 3,5-Dibromo-Nalpha-cyclohexyl-Nalpha-methyltoluene-alpha-2-diamine; Spectrum2_001526; Spectrum3_001152; Spectrum4_000758; Spectrum5_001054; BROMHEXINE [MART.]; BROMHEXINE [WHO-DD]; Oprea1_147116; SCHEMBL19059; BSPBio_002703; KBioGR_001116; KBioSS_001872; MLS004774079; MLS006011815; DivK1c_000455; SPBio_001312; CHEMBL253376; [(2-amino-3,5-dibromophenyl)methyl]cyclohexylmethylamine; DTXSID6022686; GTPL11218; KBio1_000455; KBio2_001872; KBio2_004440; KBio2_007008; KBio3_002203; OJGDCBLYJGHCIH-UHFFFAOYSA-; NINDS_000455; HMS2090K17; ZINC608220; s5943; STK177356; AKOS000305902; DB09019; IDI1_000455; NCGC00178520-01; NCGC00178520-02; NCGC00178520-05; SBI-0051773.P002; D07542; EN300-228940; AB00053644-02; AB00053644_03; AB00053644_04; A924042; Q239778; BRD-K47631482-003-02-1; BRD-K47631482-003-03-9; F2173-0412; 2,4-Dibromo-6-([cyclohexyl(methyl)amino]methyl)aniline #; 2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzenemathanamine; 2-Amino-3,5-dibromo-N-cyclohexyl-N-methyl benzenemethanamine
|
|
| CAS | 3572-43-8 | |
| PubChem CID | 2442 | |
| ChEMBL ID | CHEMBL253376 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 376.13 | ALogp: | 4.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 18 | QED Weighted: | 0.75 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.534 | MDCK Permeability: | 0.00001500 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.039 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.371 | Plasma Protein Binding (PPB): | 95.29% |
| Volume Distribution (VD): | 1.578 | Fu: | 4.10% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.407 | CYP1A2-substrate: | 0.883 |
| CYP2C19-inhibitor: | 0.433 | CYP2C19-substrate: | 0.939 |
| CYP2C9-inhibitor: | 0.019 | CYP2C9-substrate: | 0.056 |
| CYP2D6-inhibitor: | 0.991 | CYP2D6-substrate: | 0.9 |
| CYP3A4-inhibitor: | 0.037 | CYP3A4-substrate: | 0.218 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.555 | Half-life (T1/2): | 0.136 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.533 | Human Hepatotoxicity (H-HT): | 0.249 |
| Drug-inuced Liver Injury (DILI): | 0.741 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.433 | Maximum Recommended Daily Dose: | 0.946 |
| Skin Sensitization: | 0.857 | Carcinogencity: | 0.182 |
| Eye Corrosion: | 0.898 | Eye Irritation: | 0.228 |
| Respiratory Toxicity: | 0.965 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
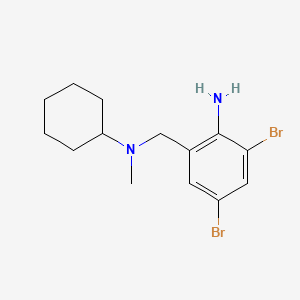
| ENC001222 | 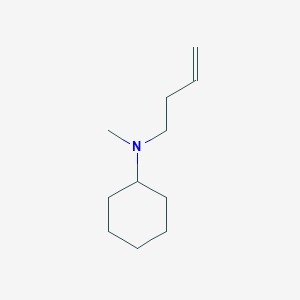 |
0.377 | D03DSR | 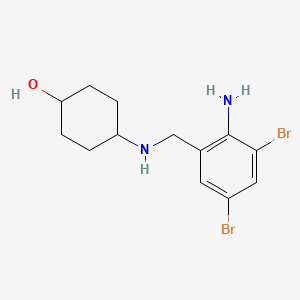 |
0.449 | ||
| ENC001186 | 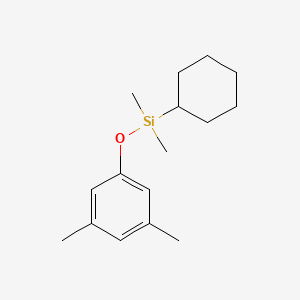 |
0.282 | D07GRH | 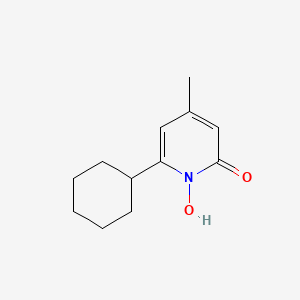 |
0.314 | ||
| ENC000492 | 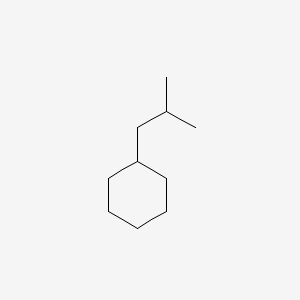 |
0.258 | D0P6VV | 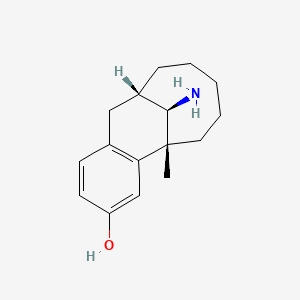 |
0.247 | ||
| ENC005003 | 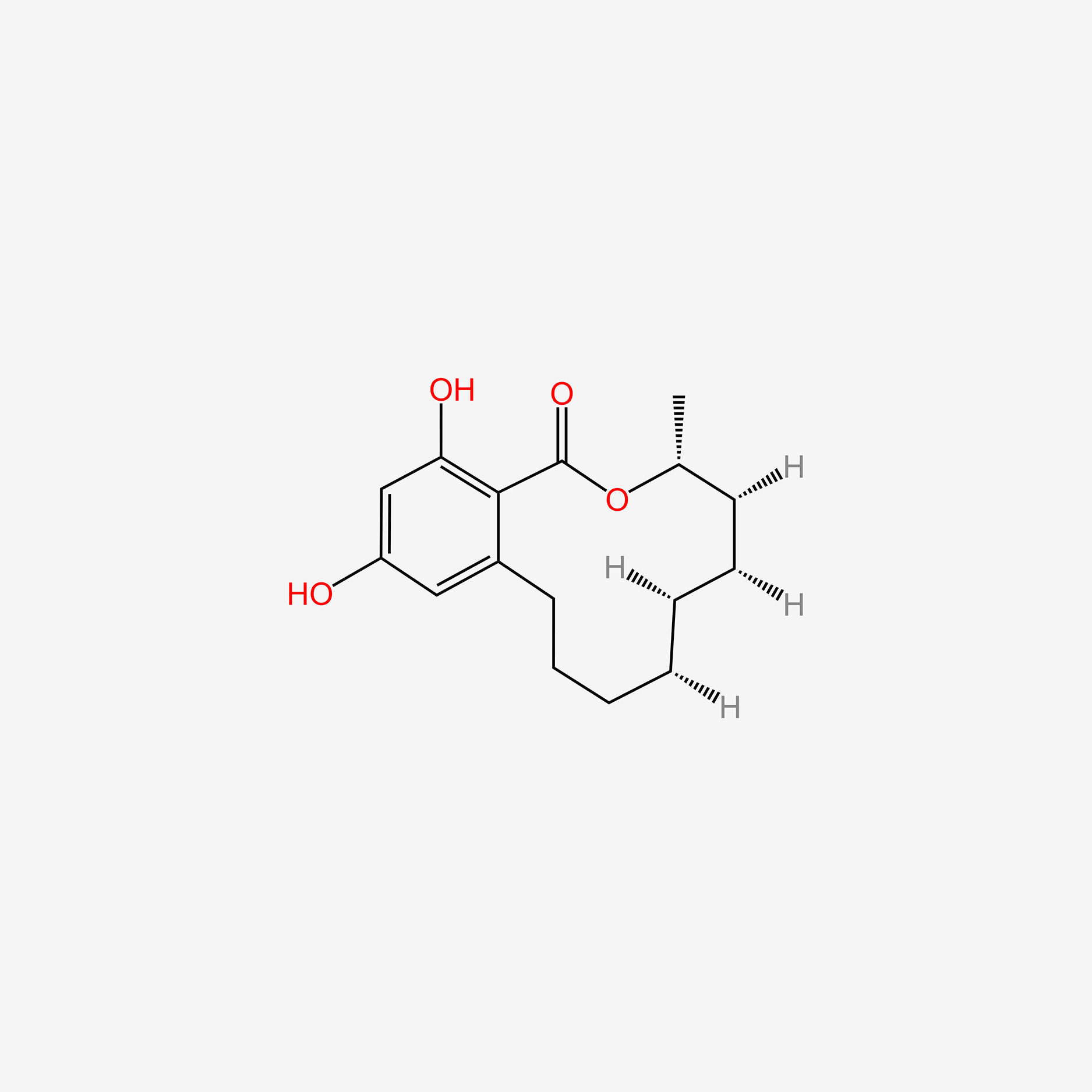 |
0.247 | D03DVJ | 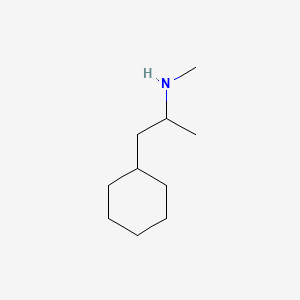 |
0.246 | ||
| ENC002297 | 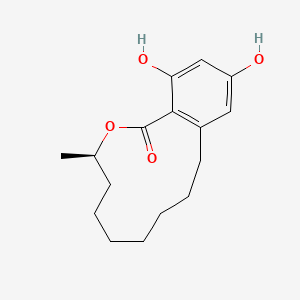 |
0.247 | D08MRN | 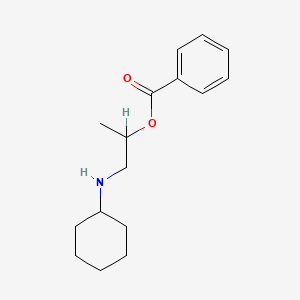 |
0.221 | ||
| ENC000644 | 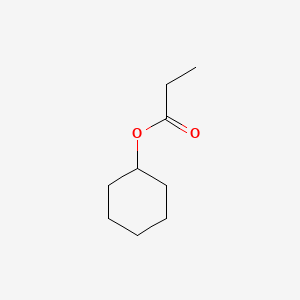 |
0.246 | D08VSI |  |
0.216 | ||
| ENC002701 |  |
0.241 | D0W1DI |  |
0.213 | ||
| ENC003244 |  |
0.241 | D05GKD | 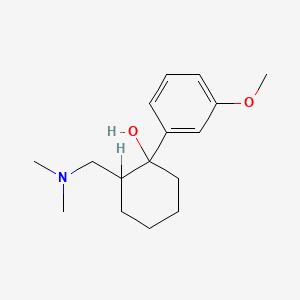 |
0.212 | ||
| ENC001430 | 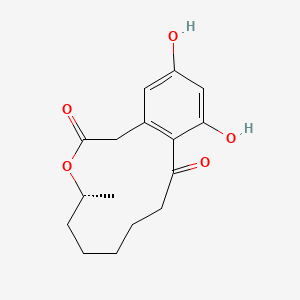 |
0.241 | D0Y2CJ | 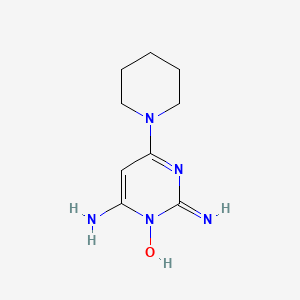 |
0.211 | ||
| ENC002059 | 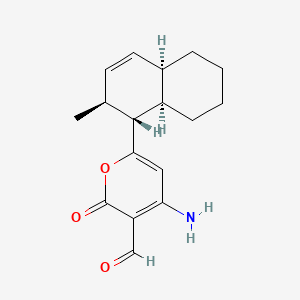 |
0.239 | D04JPJ | 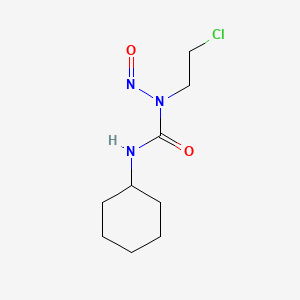 |
0.211 | ||