NPs Basic Information
|
Name |
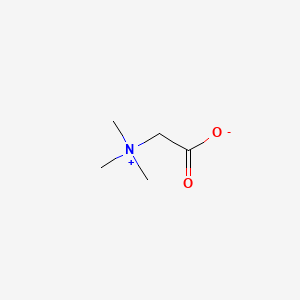
Betaine
|
| Molecular Formula | C5H11NO2 | |
| IUPAC Name* |
2-(trimethylazaniumyl)acetate
|
|
| SMILES |
C[N+](C)(C)CC(=O)[O-]
|
|
| InChI |
InChI=1S/C5H11NO2/c1-6(2,3)4-5(7)8/h4H2,1-3H3
|
|
| InChIKey |
KWIUHFFTVRNATP-UHFFFAOYSA-N
|
|
| Synonyms |
betaine; 107-43-7; glycine betaine; oxyneurine; Trimethylglycine; lycine; Abromine; Trimethylglycocoll; 2-(trimethylazaniumyl)acetate; Glycocoll betaine; Glycylbetaine; acidin-pepsin; Rubrine C; BETAINE, ANHYDROUS; Jortaine; alpha-Earleine; trimethylammonioacetate; 2-(Trimethylammonio)Acetate; N,N,N-trimethylglycine; Trimethylaminoacetic acid; Glycine, trimethylbetaine; Loramine AMB 13; (Trimethylammonio)acetate; Glykokollbetain [German]; Trimethylaminoacetate; Acidol; Methanaminium, 1-carboxy-N,N,N-trimethyl-, inner salt; Methanaminium, 1-carboxy-N,N,N-trimethyl-, hydroxide, inner salt; Betaine anhydrous; 2-trimethylammonioacetate; AI3-24187; AI3-52598; MFCD00012123; N,N,N-trimethylammonioacetate; Betafin; BRN 3537113; GLYCINEBETAINE; CHEBI:17750; (Carboxymethyl)trimethylammonium hydroxide, inner salt; 2-(Trimethylammonio)ethanoic acid, hydroxide, inner salt; 2-(trimethylamino)acetic acid; 3SCV180C9W; 1-Carboxy-N,N,N-trimethylmethanaminium hydroxide, inner salt; 2-N,N,N-trimethylammonio acetate; Aminocoat; Betaine (JAN); Greenstim; FinnStim; NSC-166511; Betafin BP; Betafin BCR; Novobetaine; .alpha.-Earleine; Hepastyl; BETAINE [JAN]; DSSTox_CID_2666; (Carboxymethyl)trimethylammonium hydroxide inner salt; DSSTox_RID_76680; DSSTox_GSID_22666; Glykokollbetain; 1-Carboxy-N,N,N-trimethylmethanaminium inner salt; FEMA NO. 4223; CAS-107-43-7; HSDB 7467; NCGC00015150-03; EINECS 203-490-6; trimethylglycocoll anhydride; NSC 166511; UNII-3SCV180C9W; Betaineanhydrous; Glycine-Betaine; .beta.ine; 3mam; 3ppp; Cystadane (TN); Betaine,(S); Betaine (8CI); Aquadew AN 100; 3l6h; BETAINE [VANDF]; BETAINE [FHFI]; BETAINE [HSDB]; BETAINE [INCI]; BETAINE [FCC]; BETAINE [MI]; BET; BETAINE [MART.]; BETAINE [USP-RS]; BETAINE [WHO-DD]; bmse000069; bmse000948; bmse000997; (trimethylammoniumyl)acetate; caprylic amidopropyl betaine; EC 203-490-6; SCHEMBL7739; CHEMBL1182; 4-04-00-02369 (Beilstein Handbook Reference); BETAINE [ORANGE BOOK]; (Carboxymethyl)trimethylammonium; GTPL4550; DTXSID8022666; BCP21888; HY-B0710; Tox21_113511; Tox21_301159; BDBM50103520; BETAINE ANHYDROUS [EMA EPAR]; NSC166511; STK372904; AKOS005206774; AM90357; CCG-266068; DB06756; SDCCGMLS-0066923.P001; NCGC00178605-01; NCGC00178605-02; NCGC00178605-03; NCGC00178605-08; NCGC00255057-01; Abromine; Lycine; Trimethylglycine (TMG); AS-12941; SY011295; Methanaminium, 1-carboxy-N,N,N-trimethyl-; B0455; FT-0622917; WLN: QV1K1 & 1 & 1 & Q; C00719; D07523; EN300-301715; AB00053634_03; A801696; methanaminium, carboxy-N,N,N-trimethyl-, inner salt; Methanaminium,N,N-trimethyl-, hydroxide, inner salt; Q-200708; Q10860583; Z2289798098; Methanaminium, 1-carboxy-N,N,N-trimethyl-, inner salt (9CI)
|
|
| CAS | 107-43-7 | |
| PubChem CID | 247 | |
| ChEMBL ID | CHEMBL1182 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 117.15 | ALogp: | 0.5 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 8 | QED Weighted: | 0.435 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.795 | MDCK Permeability: | 0.00025226 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.757 | 20% Bioavailability (F20%): | 0.552 |
| 30% Bioavailability (F30%): | 0.959 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.308 | Plasma Protein Binding (PPB): | 10.82% |
| Volume Distribution (VD): | 0.854 | Fu: | 90.87% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.008 | CYP1A2-substrate: | 0.499 |
| CYP2C19-inhibitor: | 0.017 | CYP2C19-substrate: | 0.047 |
| CYP2C9-inhibitor: | 0.016 | CYP2C9-substrate: | 0.337 |
| CYP2D6-inhibitor: | 0.004 | CYP2D6-substrate: | 0.417 |
| CYP3A4-inhibitor: | 0.004 | CYP3A4-substrate: | 0.024 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.091 | Half-life (T1/2): | 0.874 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.784 |
| Drug-inuced Liver Injury (DILI): | 0.01 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.104 | Maximum Recommended Daily Dose: | 0.224 |
| Skin Sensitization: | 0.092 | Carcinogencity: | 0.052 |
| Eye Corrosion: | 0.864 | Eye Irritation: | 0.836 |
| Respiratory Toxicity: | 0.128 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000020 |  |
0.484 | D0XB8P | 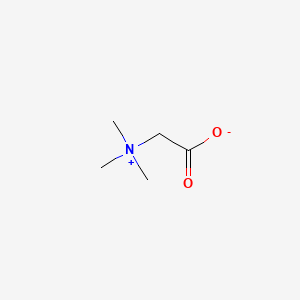 |
1.000 | ||
| ENC005538 | 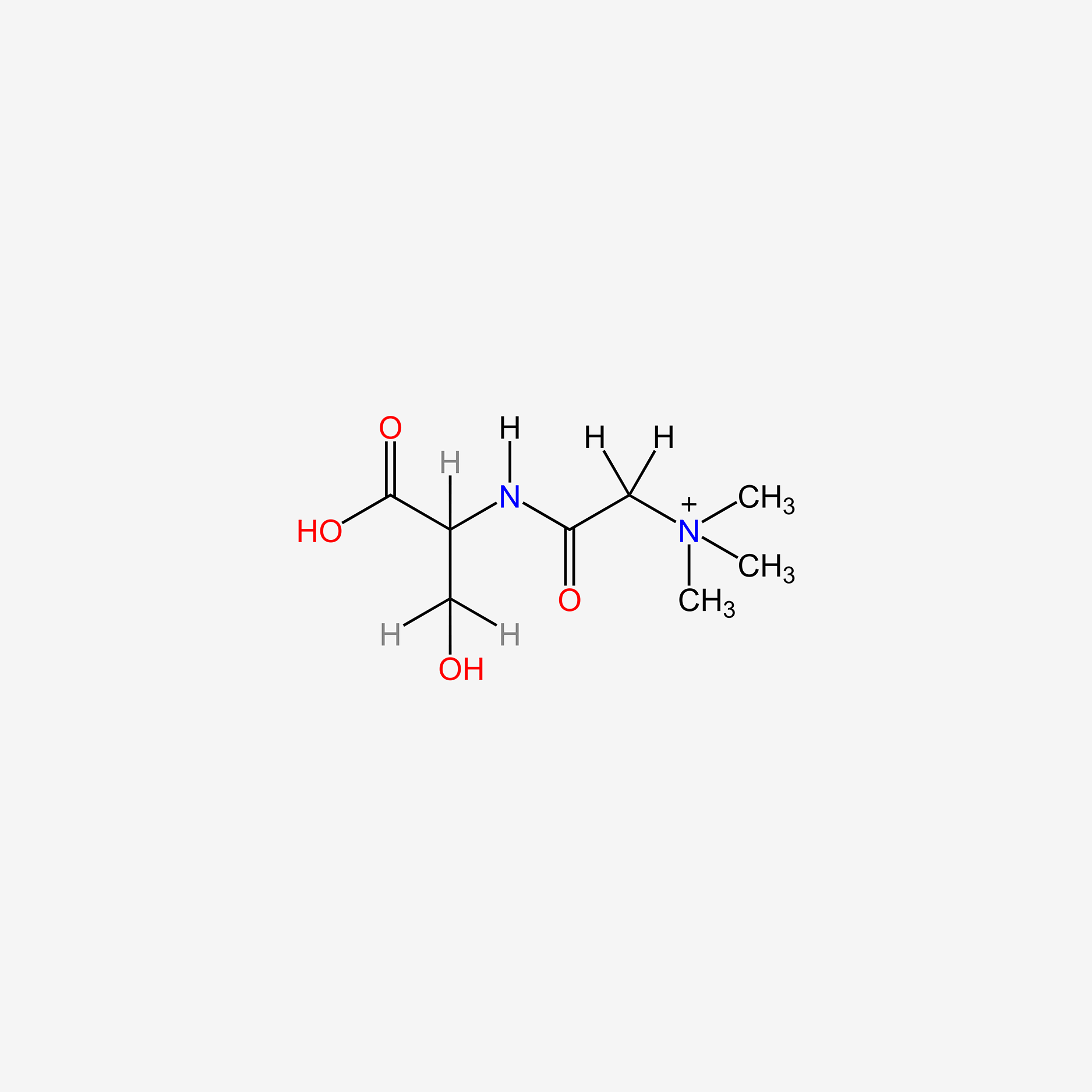 |
0.350 | D02KJX | 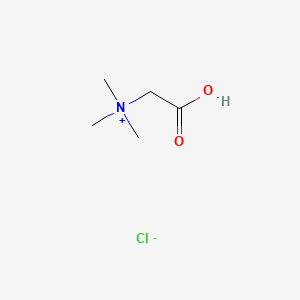 |
0.560 | ||
| ENC000008 | 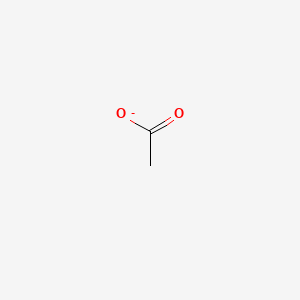 |
0.286 | D0G8SQ | 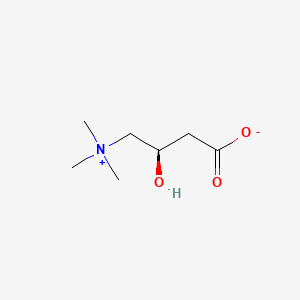 |
0.484 | ||
| ENC000237 | 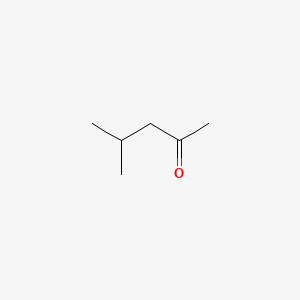 |
0.241 | D0U3IG | 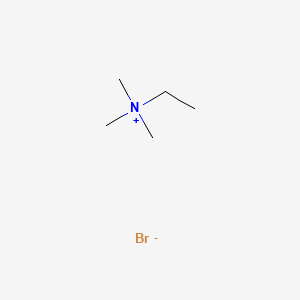 |
0.375 | ||
| ENC001610 | 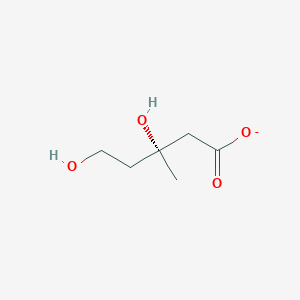 |
0.222 | D0Q9HF | 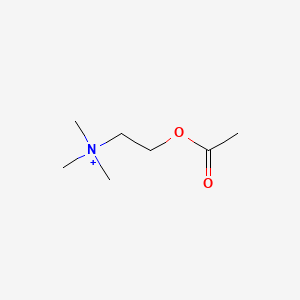 |
0.375 | ||
| ENC000246 | 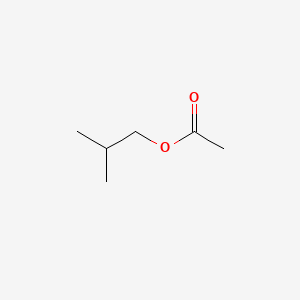 |
0.219 | D0U7BW | 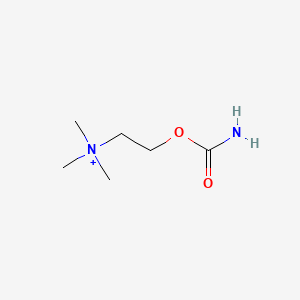 |
0.375 | ||
| ENC000225 | 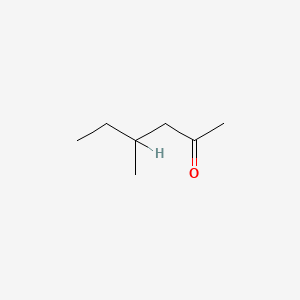 |
0.219 | D04MWJ | 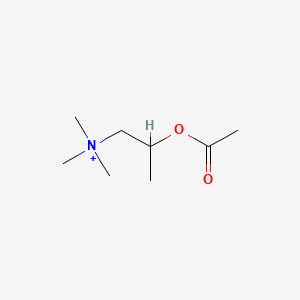 |
0.353 | ||
| ENC000186 | 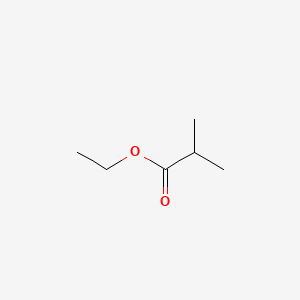 |
0.219 | D07ZTO | 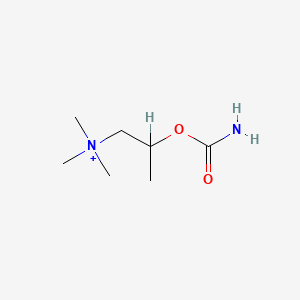 |
0.353 | ||
| ENC001900 | 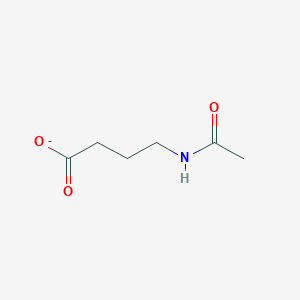 |
0.216 | D0C1QZ | 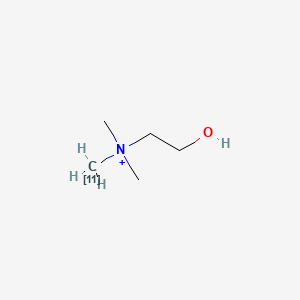 |
0.333 | ||
| ENC000312 | 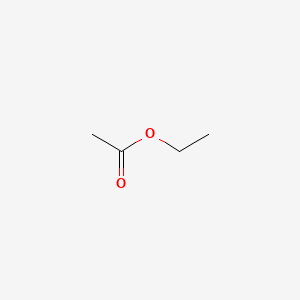 |
0.214 | D0C1PY | 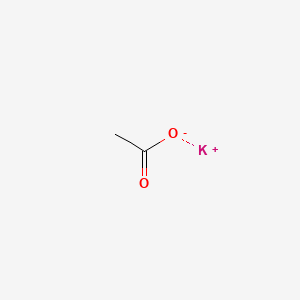 |
0.273 | ||