NPs Basic Information
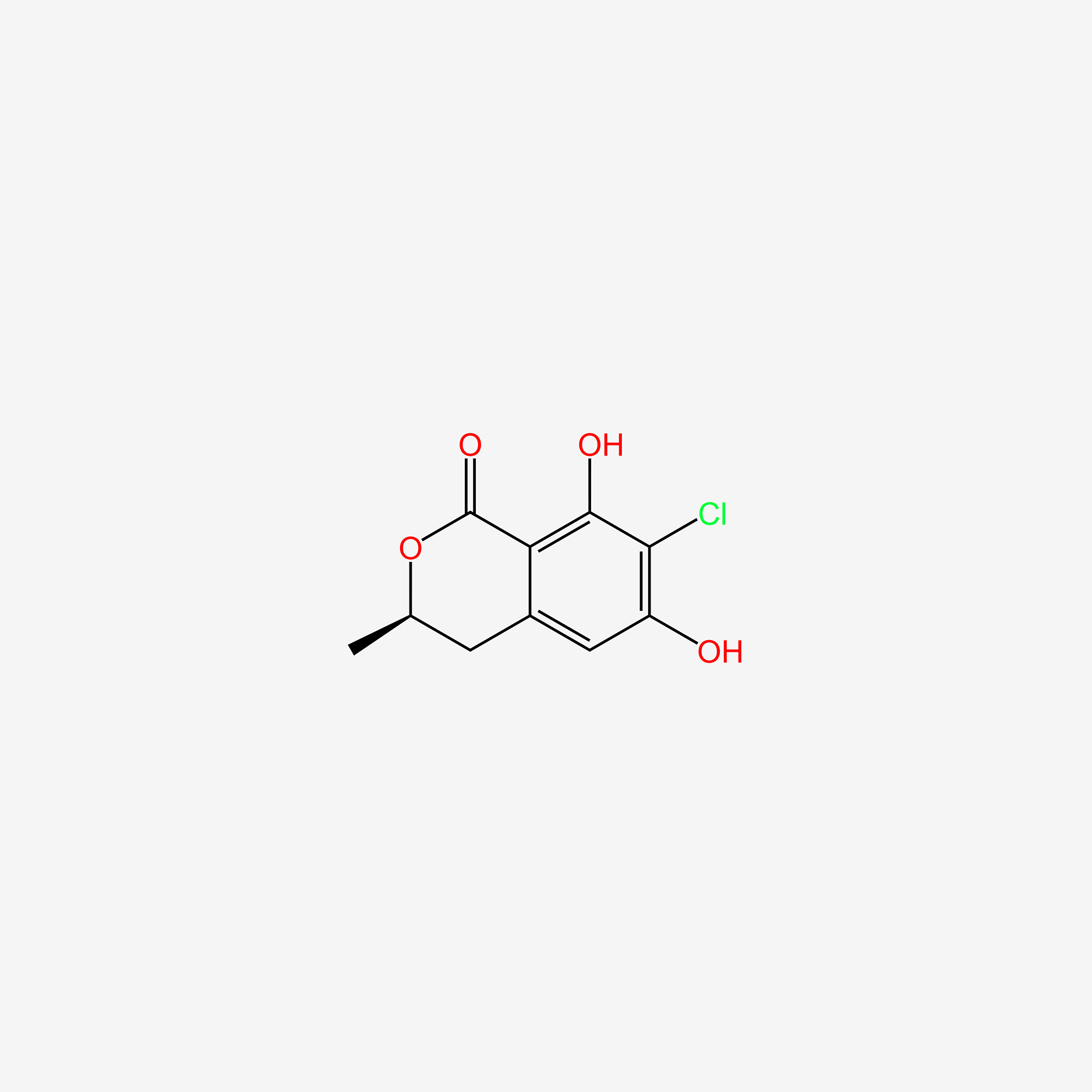
|
Name |
7-chloro-3,4-dihydro-6,8-dihydroxy-3-methylisocoumarin
|
| Molecular Formula | C10H9ClO4 | |
| IUPAC Name* |
7-chloro-6,8-dihydroxy-3-methyl-3,4-dihydroisochromen-1-one
|
|
| SMILES |
CC1Cc2cc(O)c(Cl)c(O)c2C(=O)O1
|
|
| InChI |
InChI=1S/C10H9ClO4/c1-4-2-5-3-6(12)8(11)9(13)7(5)10(14)15-4/h3-4,12-13H,2H2,1H3/t4-/m1/s1
|
|
| InChIKey |
DGSBLHFAZVSZGE-SCSAIBSYSA-N
|
|
| Synonyms |
NA
|
|
| CAS | NA | |
| PubChem CID | NA | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was calculated by STOUT. Reference: PMID:33906675.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 228.63 | ALogp: | 1.9 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 66.8 | Aromatic Rings: | 2 |
| Heavy Atoms: | 15 | QED Weighted: | 0.67 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.831 | MDCK Permeability: | 0.00002440 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.014 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.282 | Plasma Protein Binding (PPB): | 97.46% |
| Volume Distribution (VD): | 0.484 | Fu: | 2.19% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.902 | CYP1A2-substrate: | 0.444 |
| CYP2C19-inhibitor: | 0.061 | CYP2C19-substrate: | 0.101 |
| CYP2C9-inhibitor: | 0.319 | CYP2C9-substrate: | 0.754 |
| CYP2D6-inhibitor: | 0.636 | CYP2D6-substrate: | 0.284 |
| CYP3A4-inhibitor: | 0.156 | CYP3A4-substrate: | 0.146 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.189 | Half-life (T1/2): | 0.74 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.146 |
| Drug-inuced Liver Injury (DILI): | 0.902 | AMES Toxicity: | 0.036 |
| Rat Oral Acute Toxicity: | 0.089 | Maximum Recommended Daily Dose: | 0.518 |
| Skin Sensitization: | 0.445 | Carcinogencity: | 0.48 |
| Eye Corrosion: | 0.013 | Eye Irritation: | 0.906 |
| Respiratory Toxicity: | 0.734 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005553 | 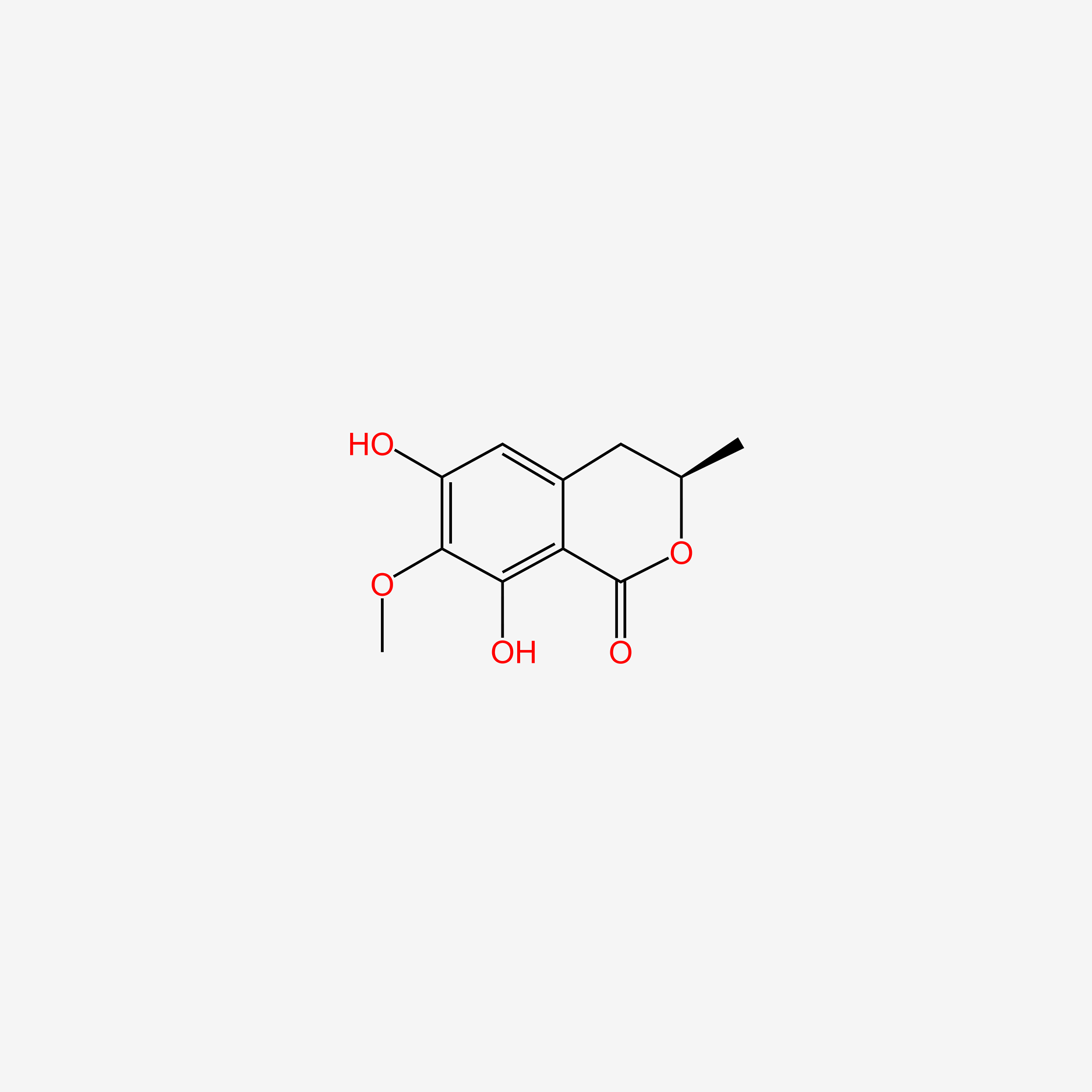 |
0.694 | D07MGA |  |
0.278 | ||
| ENC003935 |  |
0.694 | D0H6QU | 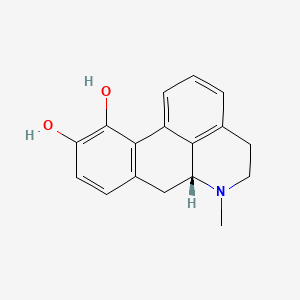 |
0.244 | ||
| ENC002045 | 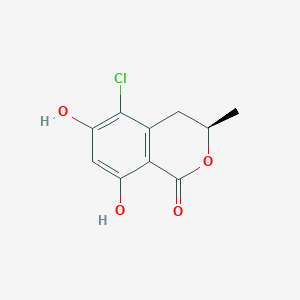 |
0.667 | D0R6BI | 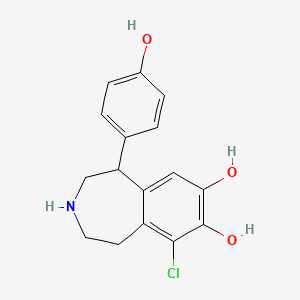 |
0.238 | ||
| ENC000960 | 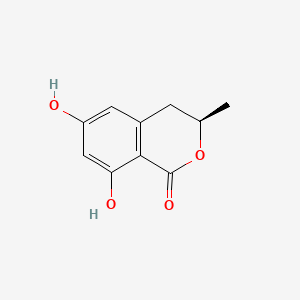 |
0.625 | D0C1SF | 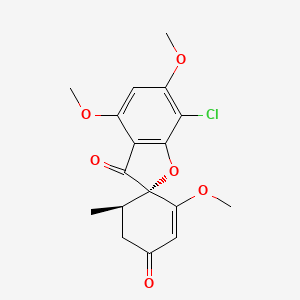 |
0.235 | ||
| ENC005249 | 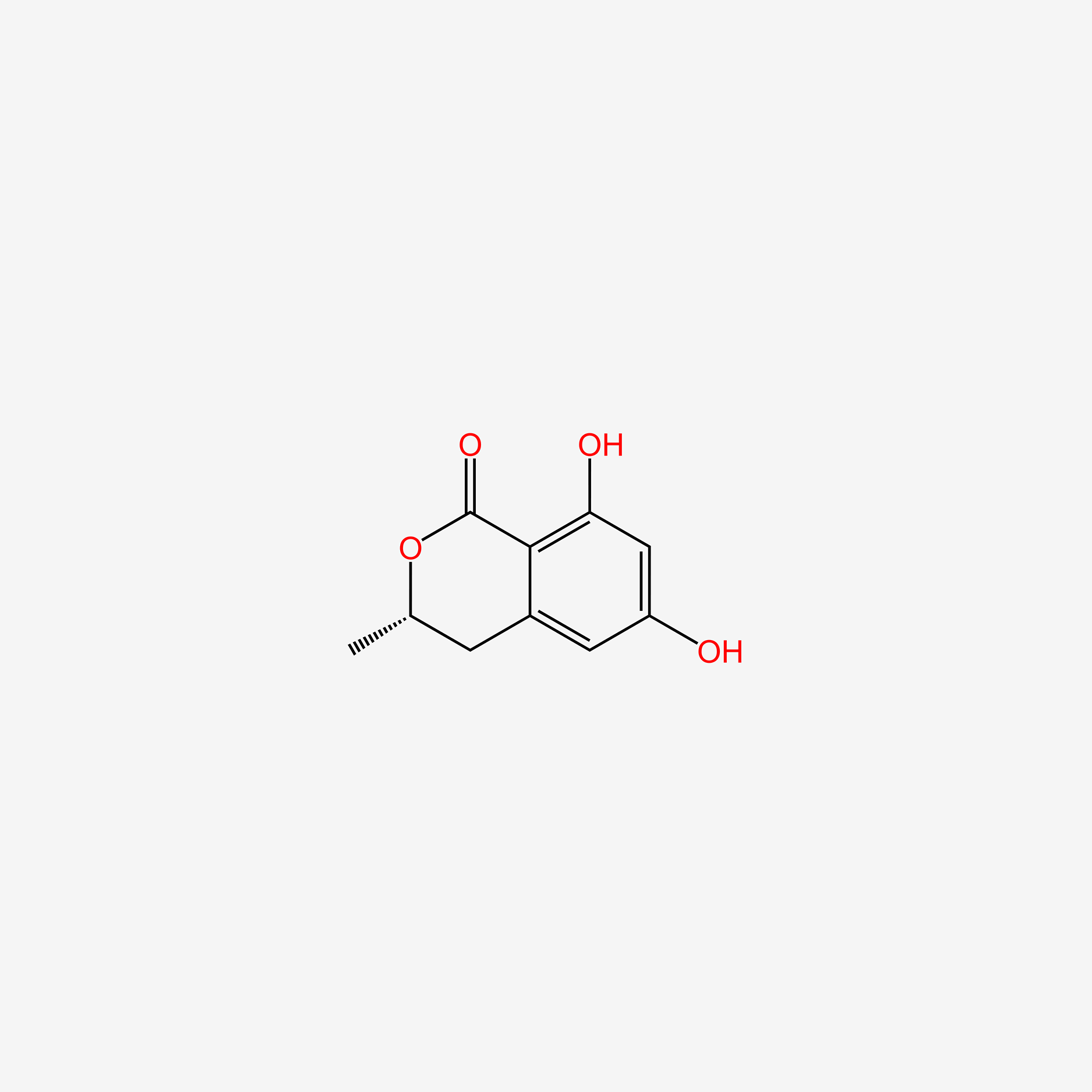 |
0.625 | D02NSF | 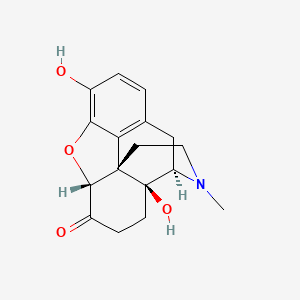 |
0.229 | ||
| ENC005248 | 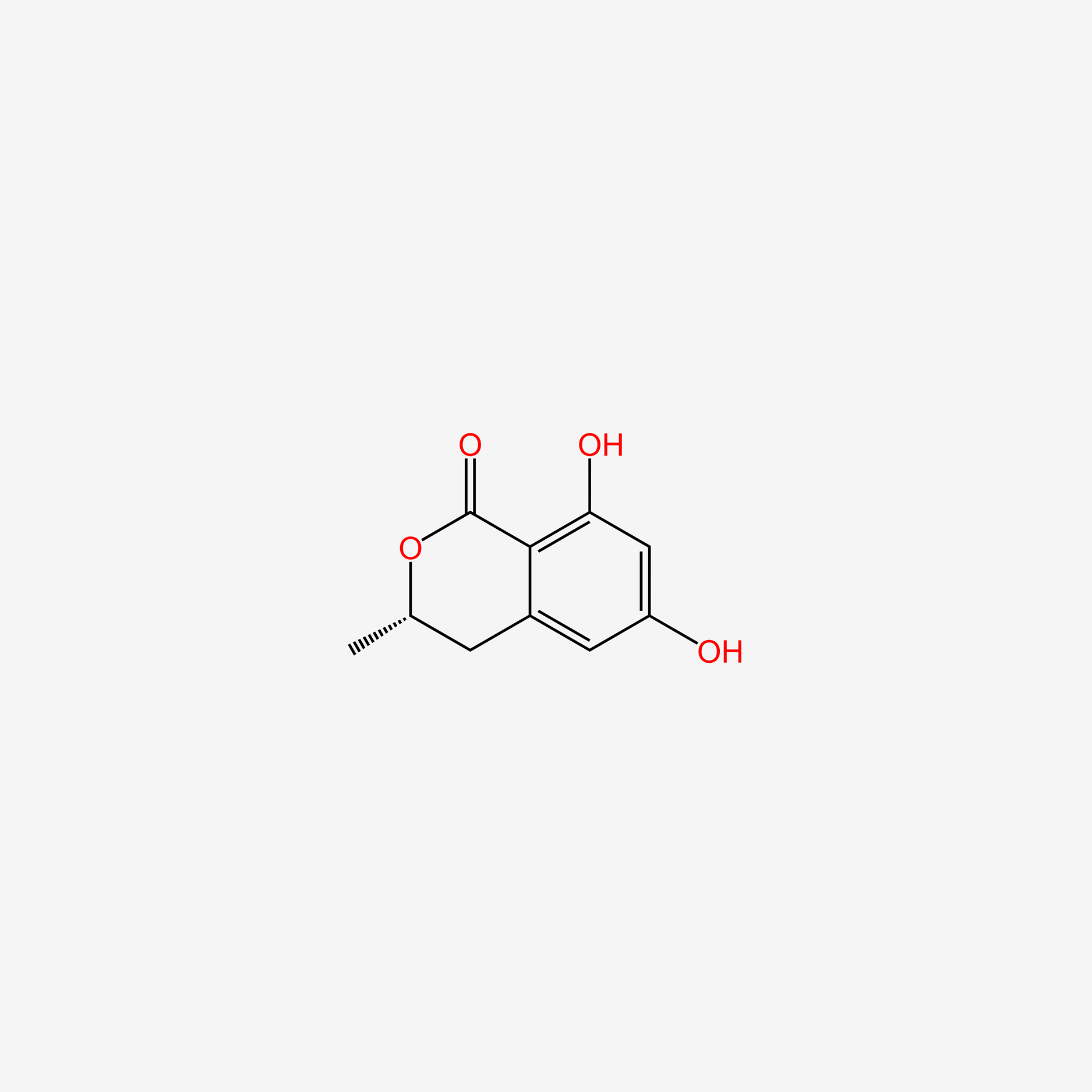 |
0.625 | D07AHW | 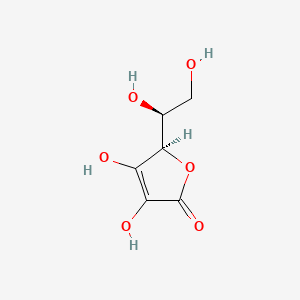 |
0.224 | ||
| ENC005706 |  |
0.577 | D04JHN |  |
0.220 | ||
| ENC003934 |  |
0.566 | D0Y7PG | 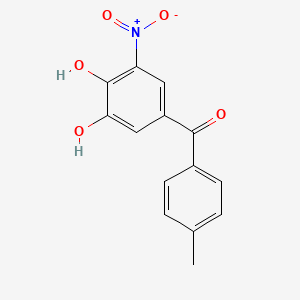 |
0.205 | ||
| ENC000757 | 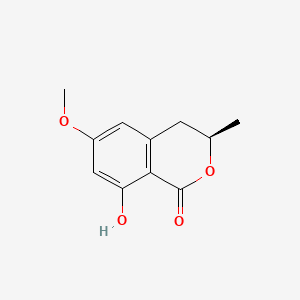 |
0.528 | D0K8KX | 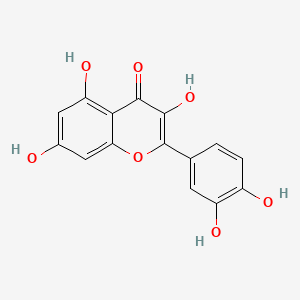 |
0.205 | ||
| ENC005939 |  |
0.500 | D0ZX2G |  |
0.203 | ||