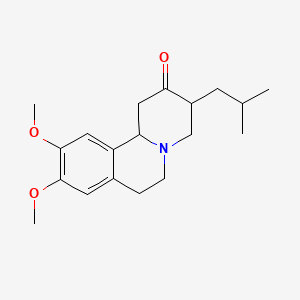
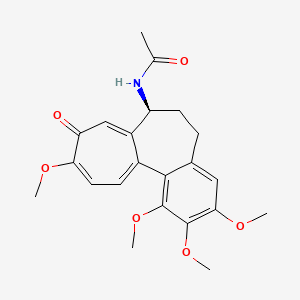
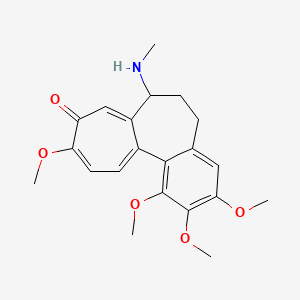
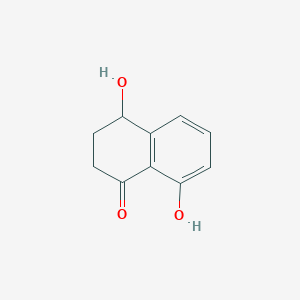
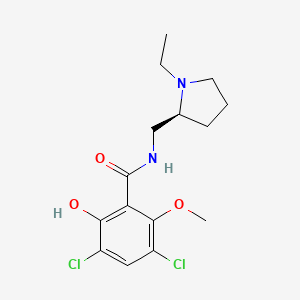
NPs Basic Information

|
Name |
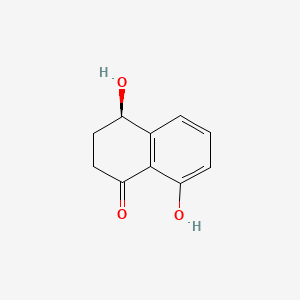
Seimatorone
|
| Molecular Formula | C12H12O5 | |
| IUPAC Name* |
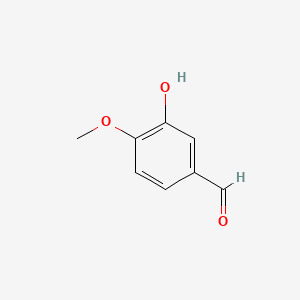
1,5-dihydroxy-3-methoxy-8-oxo-6,7-dihydro-5H-naphthalene-2-carbaldehyde
|
|
| SMILES |
COC1=C(C(=C2C(=O)CCC(C2=C1)O)O)C=O
|
|
| InChI |
InChI=1S/C12H12O5/c1-17-10-4-6-8(14)2-3-9(15)11(6)12(16)7(10)5-13/h4-5,8,14,16H,2-3H2,1H3
|
|
| InChIKey |
MSMFQQLPINTZOJ-UHFFFAOYSA-N
|
|
| Synonyms |
Seimatorone; 5,6,7,8-tetrahydro-1,5-dihydroxy-3-methoxy-8-oxonaphthalene-2-carbaldehyde
|
|
| CAS | NA | |
| PubChem CID | 137797175 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 236.22 | ALogp: | 0.5 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.8 | Aromatic Rings: | 2 |
| Heavy Atoms: | 17 | QED Weighted: | 0.763 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.022 | MDCK Permeability: | 0.00000538 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.785 | 20% Bioavailability (F20%): | 0.285 |
| 30% Bioavailability (F30%): | 0.984 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.028 | Plasma Protein Binding (PPB): | 91.30% |
| Volume Distribution (VD): | 0.5 | Fu: | 12.09% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.937 | CYP1A2-substrate: | 0.923 |
| CYP2C19-inhibitor: | 0.029 | CYP2C19-substrate: | 0.081 |
| CYP2C9-inhibitor: | 0.084 | CYP2C9-substrate: | 0.684 |
| CYP2D6-inhibitor: | 0.125 | CYP2D6-substrate: | 0.484 |
| CYP3A4-inhibitor: | 0.079 | CYP3A4-substrate: | 0.142 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.892 | Half-life (T1/2): | 0.945 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.021 |
| Drug-inuced Liver Injury (DILI): | 0.527 | AMES Toxicity: | 0.816 |
| Rat Oral Acute Toxicity: | 0.105 | Maximum Recommended Daily Dose: | 0.052 |
| Skin Sensitization: | 0.914 | Carcinogencity: | 0.28 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.919 |
| Respiratory Toxicity: | 0.259 |