NPs Basic Information
|
Name |
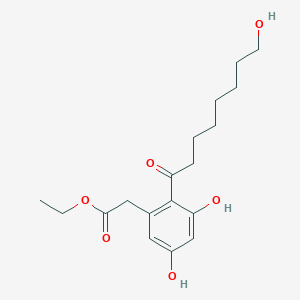
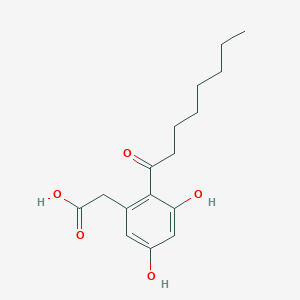
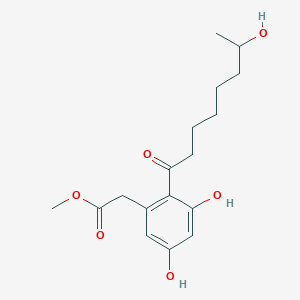
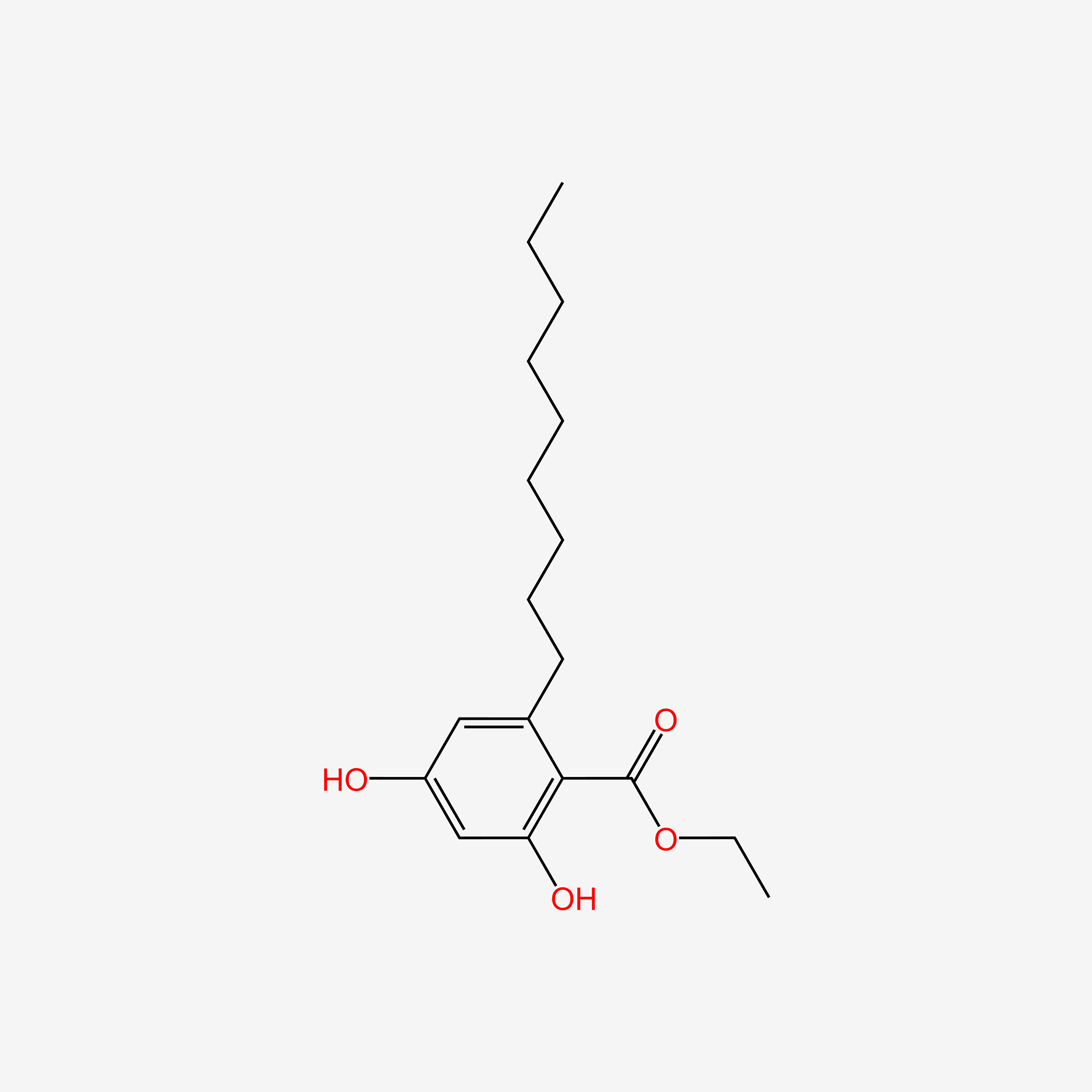
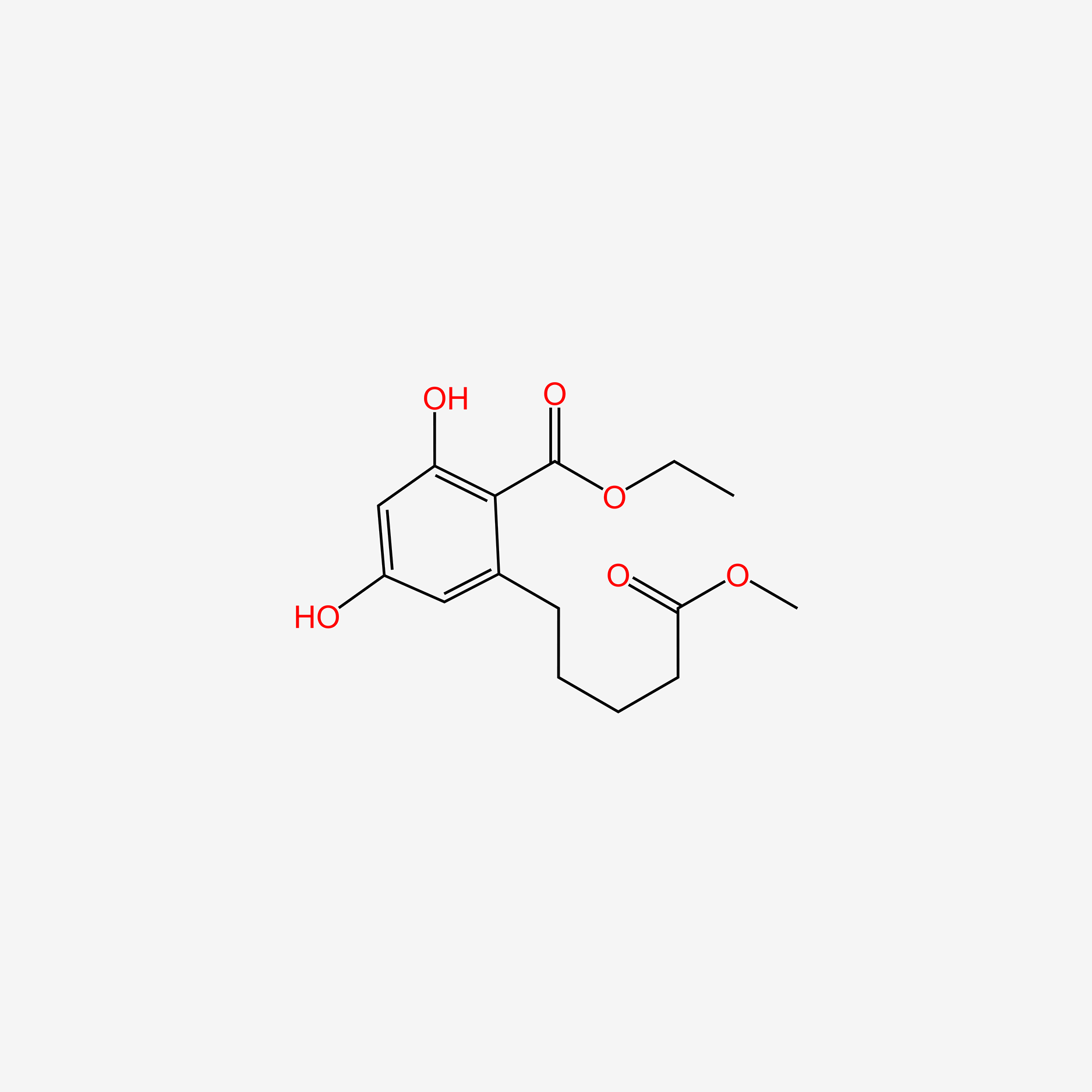
Cytosporone B
|
| Molecular Formula | C18H26O5 | |
| IUPAC Name* |
ethyl 2-(3,5-dihydroxy-2-octanoylphenyl)acetate
|
|
| SMILES |
CCCCCCCC(=O)C1=C(C=C(C=C1O)O)CC(=O)OCC
|
|
| InChI |
InChI=1S/C18H26O5/c1-3-5-6-7-8-9-15(20)18-13(11-17(22)23-4-2)10-14(19)12-16(18)21/h10,12,19,21H,3-9,11H2,1-2H3
|
|
| InChIKey |
UVVWQQKSNZLUQA-UHFFFAOYSA-N
|
|
| Synonyms |
CYTOSPORONE B; 321661-62-5; Ethyl 2-(3,5-dihydroxy-2-octanoylphenyl)acetate; Csn-B; dothiorelone G; Csn-B;Dothiorelone G; ethyl 3,5-dihydroxy-2-(1-oxooctyl)-benzeneacetate; (3,5-Dihydroxy-2-octanoyl-phenyl)-acetic acid ethyl ester; ethyl 2-[2-octanoyl-3,5-bis(oxidanyl)phenyl]ethanoate; Benzeneacetic acid, 3,5-dihydroxy-2-(1-oxooctyl)-, ethyl ester; GTPL5424; CHEMBL1221517; SCHEMBL14900697; CHEBI:95039; DTXSID80443557; 3,5-Dihydroxy-2-(1-oxooctyl)benzeneacetic acid ethyl ester; HMS3740I13; BCP18389; Cytosporone B, >=98% (HPLC); EX-A4029; HY-N2148; WMA66162; BDBM50378798; MFCD12912406; ZINC43200202; AKOS027470187; CS-6882; AC-36470; AS-16748; S6674; W16910; A935069; J-018662; BRD-K86191271-001-01-9; Cytosporone-B; Csn-B; Dothiorelone G; Dothiorelone-G; Q27076966; 3,5-Dihydroxy-2-(1-oxooctyl)benzeneacetic acid ethyl ester, Csn-B; 3,5-Dihydroxy-2-(1-oxooctyl)-benzeneacetic acid, ethyl ester, Csn-B; E4L
|
|
| CAS | 321661-62-5 | |
| PubChem CID | 10687292 | |
| ChEMBL ID | CHEMBL1221517 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 322.4 | ALogp: | 4.6 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 11 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 23 | QED Weighted: | 0.378 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.723 | MDCK Permeability: | 0.00002620 |
| Pgp-inhibitor: | 0.041 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.99 |
| 30% Bioavailability (F30%): | 0.856 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.348 | Plasma Protein Binding (PPB): | 94.34% |
| Volume Distribution (VD): | 0.674 | Fu: | 7.06% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.972 | CYP1A2-substrate: | 0.379 |
| CYP2C19-inhibitor: | 0.959 | CYP2C19-substrate: | 0.085 |
| CYP2C9-inhibitor: | 0.898 | CYP2C9-substrate: | 0.949 |
| CYP2D6-inhibitor: | 0.888 | CYP2D6-substrate: | 0.148 |
| CYP3A4-inhibitor: | 0.843 | CYP3A4-substrate: | 0.149 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.856 | Half-life (T1/2): | 0.87 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.043 | Human Hepatotoxicity (H-HT): | 0.077 |
| Drug-inuced Liver Injury (DILI): | 0.713 | AMES Toxicity: | 0.537 |
| Rat Oral Acute Toxicity: | 0.129 | Maximum Recommended Daily Dose: | 0.035 |
| Skin Sensitization: | 0.806 | Carcinogencity: | 0.098 |
| Eye Corrosion: | 0.012 | Eye Irritation: | 0.907 |
| Respiratory Toxicity: | 0.167 |