NPs Basic Information
|
Name |
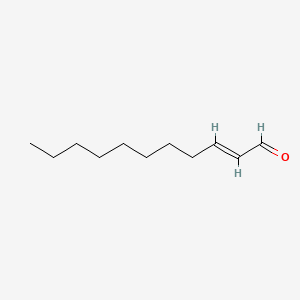
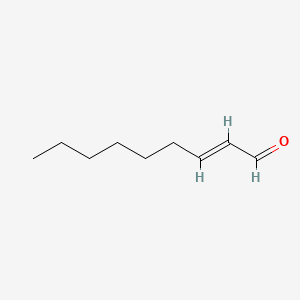
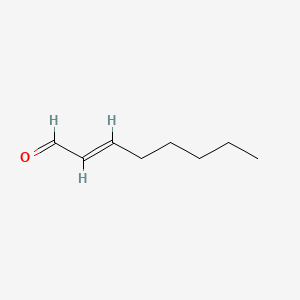
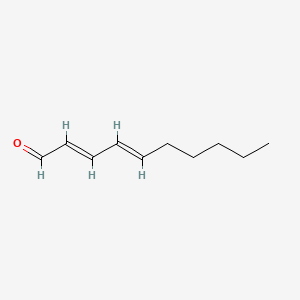
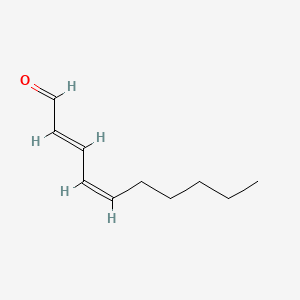
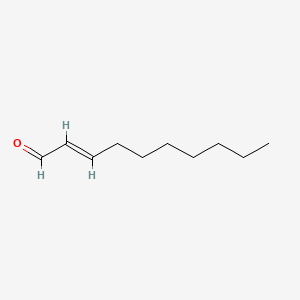
2-Decenal
|
| Molecular Formula | C10H18O | |
| IUPAC Name* |
(E)-dec-2-enal
|
|
| SMILES |
CCCCCCC/C=C/C=O
|
|
| InChI |
InChI=1S/C10H18O/c1-2-3-4-5-6-7-8-9-10-11/h8-10H,2-7H2,1H3/b9-8+
|
|
| InChIKey |
MMFCJPPRCYDLLZ-CMDGGOBGSA-N
|
|
| Synonyms |
trans-2-Decenal; (E)-Dec-2-enal; 3913-81-3; decenal; 2-DECENAL; (E)-2-Decenal; 3-Heptylacrolein; 2-Decenal, (2E)-; Decenaldehyde; 2-Decen-1-al; 3913-71-1; Decylenic aldehyde; (2E)-2-Decenal; 2-Decenal, (E)-; trans-Dec-2-enal; FEMA No. 2366; dec-2-enal; 25447-70-5; TRANS-2-DECEN-1-AL; E93S23U2BU; MFCD00014679; TRANS-2-DECEN-1-AL 10% IN ETHANOL; 2-decenaldehyde; 3-heptyl-acrolein; (2E)-decenal; EINECS 223-472-1; NSC 20747; CHEBI:61727; UNII-E93S23U2BU; AI3-36267; NSC-20747; 2-decanal; 2(e)-decenal; EINECS 223-474-2; dec-(e)-2-enal; Dec-2(E)-enal; (2E)-dec-2-enal; Dec-2-enal, (E); (e)-2-decen-1-al; trans-2-Decenyl Aldehyde; (2E)-2-Decenal #; t-2-DCA; (E)-dec-2-en-1-al; 2-DECENAL [FHFI]; DSSTox_CID_27035; DSSTox_RID_82056; DSSTox_GSID_47035; DECENALDEHYDE, TRANS-; SCHEMBL872778; CHEMBL507518; trans-2-Decen-1-al,>93%; DTXSID5047035; (E)-2-DECENAL [FCC]; FEMA 2366; CHEBI:133455; ZINC1571216; Tox21_302302; LMFA06000053; trans-2-Decenal, analytical standard; AKOS015839092; trans-2-Decenal, >=95.0% (GC); trans-2-Decenal, >=95%, FCC, FG; NCGC00256196-01; AS-44363; LS-13867; trans-2-decen-1-al FCC, No Antioxidant; CAS-3913-81-3; D1406; D1642; A873709; Q27277030; trans-2-Decen-1-al (contains trans-2-decen-1-al diethyl acetal); trans-2-Decen-1-al (contaisn trans-2-decen-1-al diethyl acetal) (10% in ethanol); trans-2-Decenal
|
|
| CAS | 3913-81-3 | |
| PubChem CID | 5283345 | |
| ChEMBL ID | CHEMBL507518 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 154.25 | ALogp: | 3.7 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 11 | QED Weighted: | 0.309 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.379 | MDCK Permeability: | 0.00003050 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.682 |
| 30% Bioavailability (F30%): | 0.969 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.997 | Plasma Protein Binding (PPB): | 85.57% |
| Volume Distribution (VD): | 0.856 | Fu: | 16.58% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.954 | CYP1A2-substrate: | 0.687 |
| CYP2C19-inhibitor: | 0.714 | CYP2C19-substrate: | 0.793 |
| CYP2C9-inhibitor: | 0.37 | CYP2C9-substrate: | 0.963 |
| CYP2D6-inhibitor: | 0.089 | CYP2D6-substrate: | 0.855 |
| CYP3A4-inhibitor: | 0.077 | CYP3A4-substrate: | 0.142 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.504 | Half-life (T1/2): | 0.5 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.042 | Human Hepatotoxicity (H-HT): | 0.046 |
| Drug-inuced Liver Injury (DILI): | 0.08 | AMES Toxicity: | 0.569 |
| Rat Oral Acute Toxicity: | 0.05 | Maximum Recommended Daily Dose: | 0.056 |
| Skin Sensitization: | 0.955 | Carcinogencity: | 0.653 |
| Eye Corrosion: | 0.982 | Eye Irritation: | 0.986 |
| Respiratory Toxicity: | 0.945 |