NPs Basic Information
|
Name |
Vomifoliol
|
| Molecular Formula | C13H20O3 | |
| IUPAC Name* |
(4S)-4-hydroxy-4-[(E,3R)-3-hydroxybut-1-enyl]-3,5,5-trimethylcyclohex-2-en-1-one
|
|
| SMILES |
CC1=CC(=O)CC([C@]1(/C=C/[C@@H](C)O)O)(C)C
|
|
| InChI |
InChI=1S/C13H20O3/c1-9-7-11(15)8-12(3,4)13(9,16)6-5-10(2)14/h5-7,10,14,16H,8H2,1-4H3/b6-5+/t10-,13-/m1/s1
|
|
| InChIKey |
KPQMCAKZRXOZLB-KOIHBYQTSA-N
|
|
| Synonyms |
Vomifoliol; 23526-45-6; (6S,9R)-vomifoliol; BLUMENOL A; Roseoside aglycon; (6S,9R)-6-hydroxy-3-oxo-alpha-ionol; (+/-)-Volifoliol; (+/-)-Blumenol-A; (+)-Vomifoliol; CHEBI:49164; (4S)-4-hydroxy-4-[(E,3R)-3-hydroxybut-1-enyl]-3,5,5-trimethylcyclohex-2-en-1-one; B7QV234K84; P86438KC5J; (6S,7E,9R)-6,9-dihydroxy-4,7-megastigmadien-3-one; FEMA no. 4661, (4S,3R)-(E)-(+/-)-; 2-Cyclohexen-1-one, 4-hydroxy-4-((1E,3R)-3-hydroxy-1-buten-1-yl)-3,5,5-trimethyl-, (4S)-; 2-Cyclohexen-1-one, 4-hydroxy-4-((1E,3R)-3-hydroxy-1-butenyl)-3,5,5-trimethyl-, (4S)-; 2-Cyclohexen-1-one, 4-hydroxy-4-(3-hydroxy-1-butenyl)-3,5,5-trimethyl-, (R*,S*-(E))-; 2-Cyclohexen-1-one, 4-hydroxy-4-(3-hydroxy-1-butenyl)-3,5,5-trimethyl-, (S-(R*,S*-(E)))-; 4-Hydroxy-4-(3-hydroxy-1-butenyl)-3,5,5-trimethyl-2-cyclohexen-1-one, (4S,3R)-(E)-(+/-)-; 2-Cyclohexen-1-one, 4-hydroxy-4-((1E,3R)-3-hydroxy-1-buten-1-yl)-3,5,5-trimethyl-, (4S)-rel-; 2-Cyclohexen-1-one, 4-hydroxy-4-(3-hydroxy-1-butenyl)-3,5,5-trimethyl-, (R*,S*-(E))-(+/-)-; 50763-73-0; (4S)-4-hydroxy-4-[(1E,3R)-3-hydroxybut-1-enyl]-3,5,5-trimethylcyclohex-2-en-1-one; (+-)-Vomifoliol; Vomifoliol, (+)-; (+-)-Blumenol A; Vomifoliol, (+-)-; UNII-B7QV234K84; UNII-P86438KC5J; (+)-BLUMENOL A; CHEMBL463088; SCHEMBL1243896; DTXSID601009964; HY-N1077; ZINC4095719; (+-)-6-hydroxy-3-oxo-alpha-ionol; BDBM50463336; NSC805019; AKOS032948398; NSC-805019; 2-Cyclohexen-1-one, 4-hydroxy-4-(3-hydroxy-1-butenyl)-3,5,5-trimethyl-, (+-)-; CS-0016364; C01760; (6S,9R)-6-HYDROXY-3-OXO-.ALPHA.-IONOL; Q22911785; (4S)-4-hydroxy-4-[(1E,3R)-3-hydroxybut-1-en-1-yl]-3,5,5-trimethylcyclohex-2-en-1-one; 2-CYCLOHEXEN-1-ONE, 4-HYDROXY-4-(3-HYDROXY-1-BUTENYL)-3,5,5-TRIMETHYL-, (+)-
|
|
| CAS | 23526-45-6 | |
| PubChem CID | 5280462 | |
| ChEMBL ID | CHEMBL463088 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 224.3 | ALogp: | 0.5 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 16 | QED Weighted: | 0.707 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.654 | MDCK Permeability: | 0.00002260 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.098 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.665 | Plasma Protein Binding (PPB): | 74.50% |
| Volume Distribution (VD): | 0.858 | Fu: | 24.29% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.023 | CYP1A2-substrate: | 0.407 |
| CYP2C19-inhibitor: | 0.1 | CYP2C19-substrate: | 0.849 |
| CYP2C9-inhibitor: | 0.042 | CYP2C9-substrate: | 0.2 |
| CYP2D6-inhibitor: | 0.002 | CYP2D6-substrate: | 0.11 |
| CYP3A4-inhibitor: | 0.017 | CYP3A4-substrate: | 0.753 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.999 | Half-life (T1/2): | 0.537 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.03 | Human Hepatotoxicity (H-HT): | 0.418 |
| Drug-inuced Liver Injury (DILI): | 0.169 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.822 | Maximum Recommended Daily Dose: | 0.966 |
| Skin Sensitization: | 0.457 | Carcinogencity: | 0.744 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.09 |
| Respiratory Toxicity: | 0.97 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
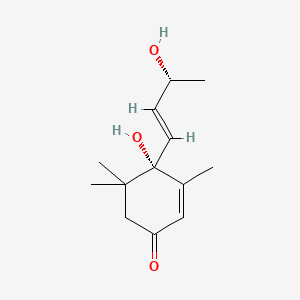
| ENC002418 | 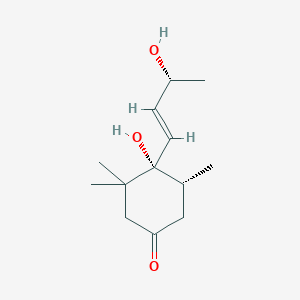 |
0.491 | D04GJN | 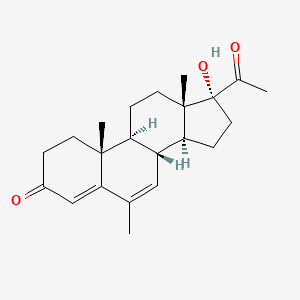 |
0.211 | ||
| ENC000146 | 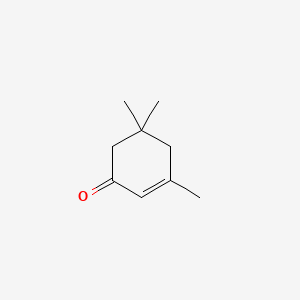 |
0.340 | D04ATM | 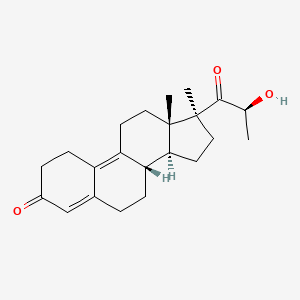 |
0.209 | ||
| ENC005579 | 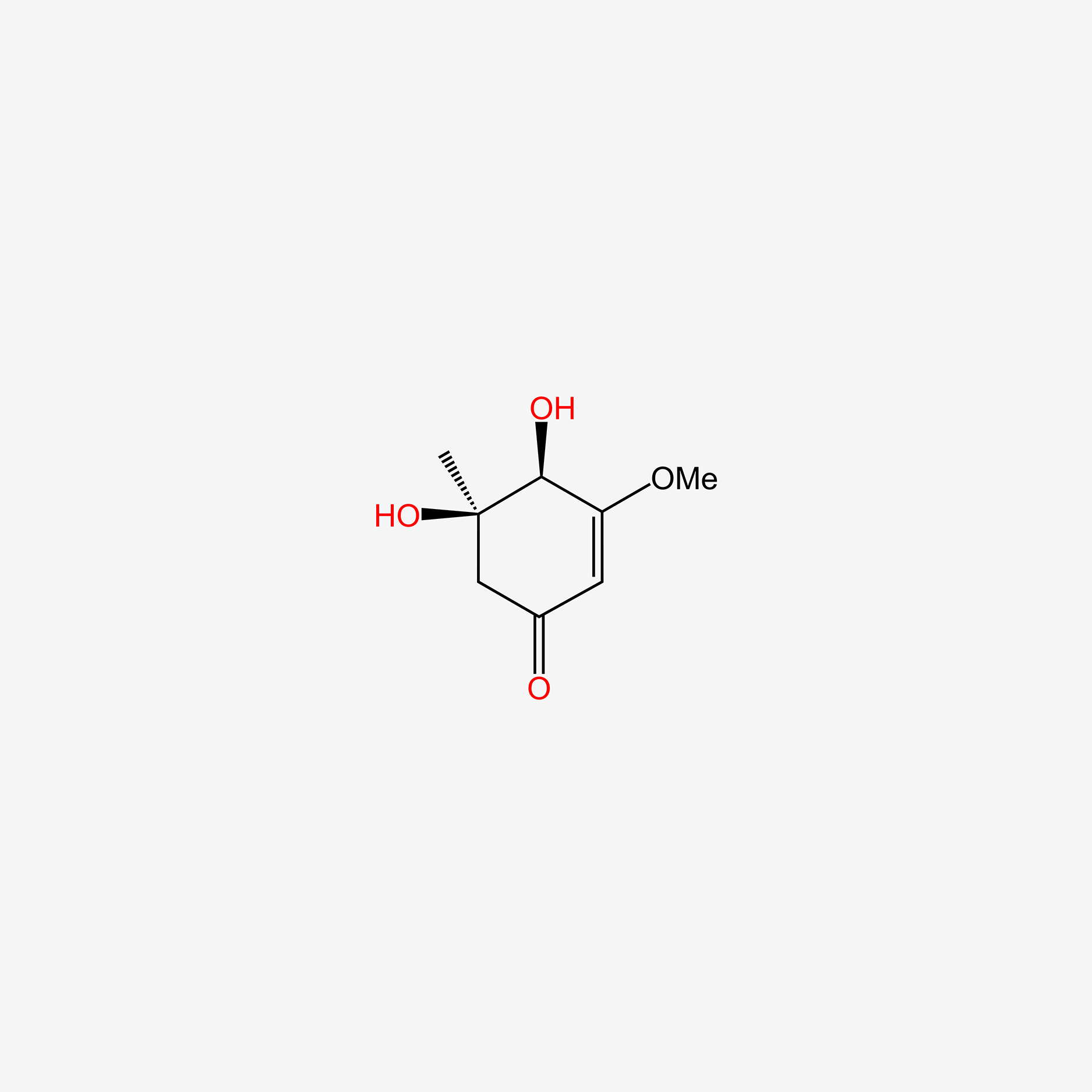 |
0.309 | D0L2LS | 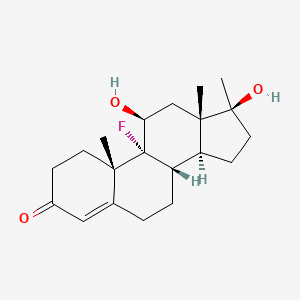 |
0.205 | ||
| ENC001370 | 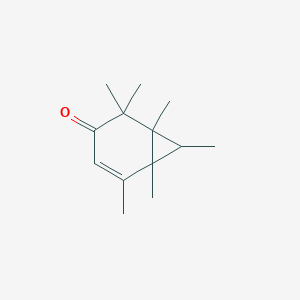 |
0.267 | D0H6VY |  |
0.203 | ||
| ENC004213 | 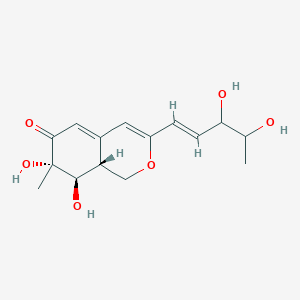 |
0.260 | D0Z1XD | 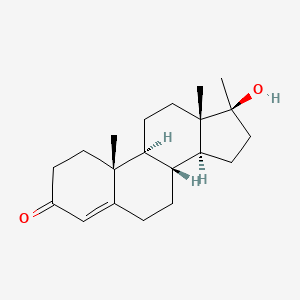 |
0.200 | ||
| ENC004208 | 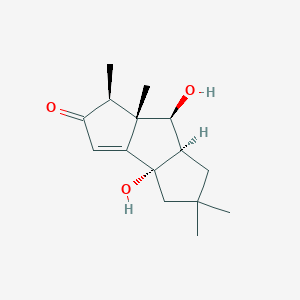 |
0.257 | D0I2SD | 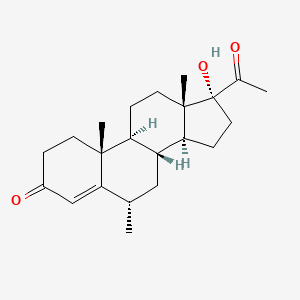 |
0.198 | ||
| ENC004209 | 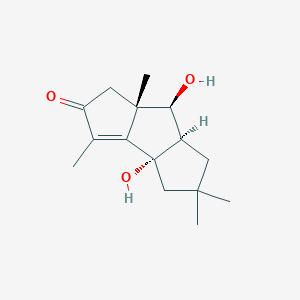 |
0.257 | D0IX6I | 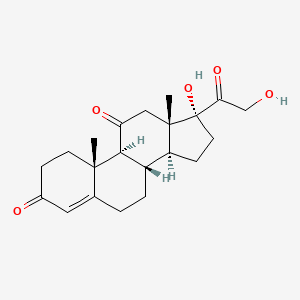 |
0.191 | ||
| ENC006101 | 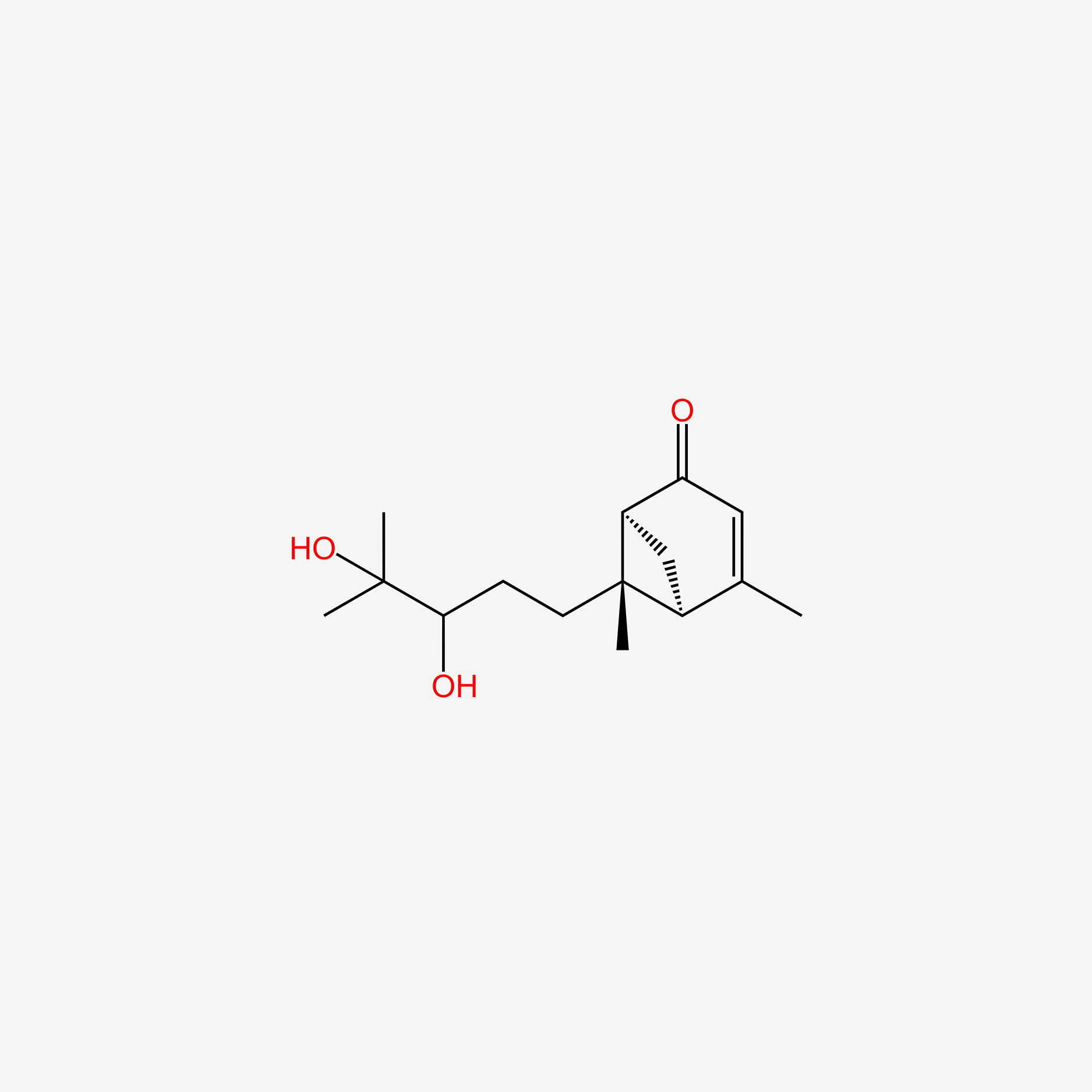 |
0.257 | D0P0HT | 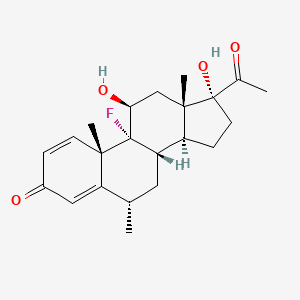 |
0.189 | ||
| ENC002957 | 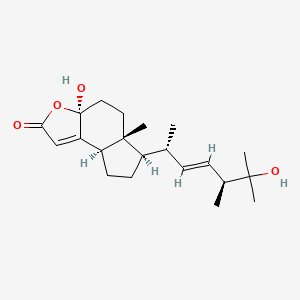 |
0.256 | D0Q6NZ | 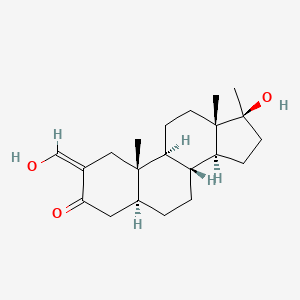 |
0.189 | ||
| ENC004904 | 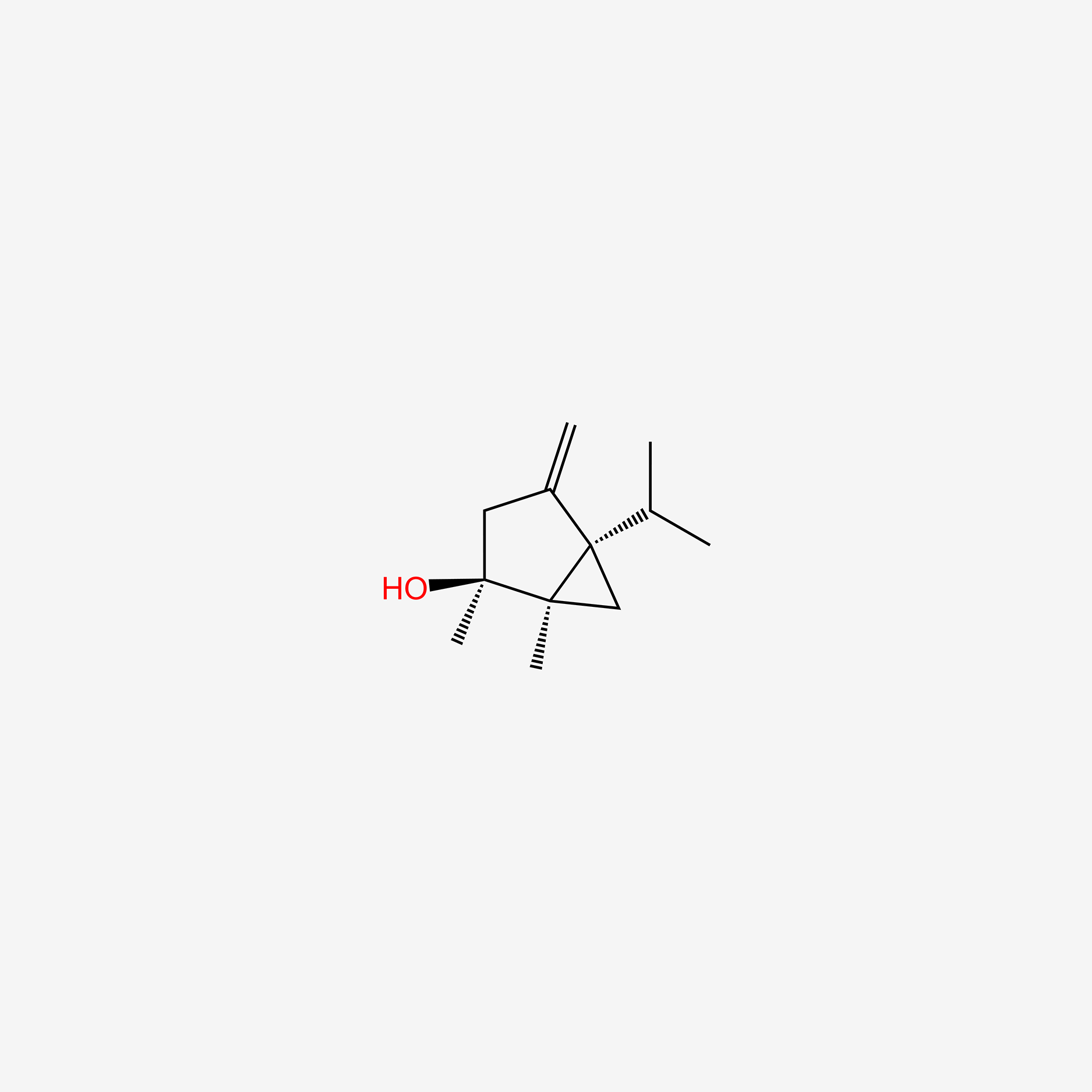 |
0.254 | D0I5DS | 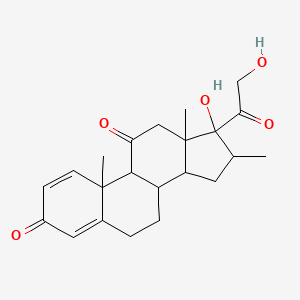 |
0.188 | ||