NPs Basic Information
|
Name |
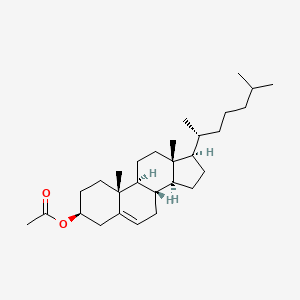
Cholesteryl acetate
|
| Molecular Formula | C29H48O2 | |
| IUPAC Name* |
[(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate
|
|
| SMILES |
C[C@H](CCCC(C)C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)OC(=O)C)C)C
|
|
| InChI |
InChI=1S/C29H48O2/c1-19(2)8-7-9-20(3)25-12-13-26-24-11-10-22-18-23(31-21(4)30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-20,23-27H,7-9,11-18H2,1-6H3/t20-,23+,24+,25-,26+,27+,28+,29-/m1/s1
|
|
| InChIKey |
XUGISPSHIFXEHZ-VEVYEIKRSA-N
|
|
| Synonyms |
Cholesteryl acetate; 604-35-3; Cholesterol acetate; 3b-Acetoxycholest-5-ene; Cholesterin acetate; 3beta-Acetoxycholest-5-ene; Cholesterol 3-acetate; (-)-Cholesteryl acetate; Cholest-5-en-3beta-ol acetate; Cholest-5-en-3beta-yl acetate; 3-Cholesteryl acetate; Cholesterylacetate; OTA9A3781T; CHEBI:78242; Cholesterol, acetate; (3beta)-cholest-5-en-3-yl acetate; Cholest-5-en-3-beta-yl acetate; NSC 8799; MFCD00003636; Cholest-5-en-3-ol (3.beta.)-, acetate; Acetic Acid Cholesterol Ester; Cholest-5-en-3-yl acetate #; UNII-OTA9A3781T; NSC-8799; CCRIS 5350; Acetyl Cholesterol; EINECS 210-066-4; 3a-Cholesterol acetate; Cholesterol 3b-acetate; AI3-24120; cholesterol 3beta-acetate; Cholesteryl acetate, 97%; SCHEMBL25655; Cholest-5-en-3b-ol acetate; Cholest-5-en-3b-yl acetate; CHEMBL3138728; DTXSID60889358; CHOLESTERYL ACETATE [INCI]; (3b)-Cholest-5-en-3-ol acetate; CHOLESTEROL 3.BETA.-ACETATE; ZINC3861169; (3beta)-cholest-5-en-3-ol acetate; LMST01010245; s4968; ACETIC ACID, CHOLESTEROL ESTER; AKOS015955666; Cholest-5-en-3-ol (3beta)-, acetate; s10712; (3I(2))-cholest-5-en-3-yl ethanoate; [(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate; AS-57181; Cholest-5-en-3-ol (3beta)-, 3-acetate; CS-0030699; Cholest-5-en-3-ol (3.beta.)-, 3-acetate; A868978; CHOLEST-5-ENE-3-OL (3.BETA.)-, ACETATE; W-105252; Q27147700; (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate; (3S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate; [(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]acetate; cholest-5-en-3-yl acetate; Chloesteryl acetate; Acetic acid cholesteryl ester; Cholesterol Acetate
|
|
| CAS | 604-35-3 | |
| PubChem CID | 2723897 | |
| ChEMBL ID | CHEMBL3138728 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 428.7 | ALogp: | 9.1 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 4 |
| Heavy Atoms: | 31 | QED Weighted: | 0.304 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.612 | MDCK Permeability: | 0.00001750 |
| Pgp-inhibitor: | 0.986 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.001 | 20% Bioavailability (F20%): | 0.902 |
| 30% Bioavailability (F30%): | 0.08 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.106 | Plasma Protein Binding (PPB): | 92.79% |
| Volume Distribution (VD): | 1.464 | Fu: | 1.45% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.083 | CYP1A2-substrate: | 0.154 |
| CYP2C19-inhibitor: | 0.183 | CYP2C19-substrate: | 0.728 |
| CYP2C9-inhibitor: | 0.259 | CYP2C9-substrate: | 0.373 |
| CYP2D6-inhibitor: | 0.232 | CYP2D6-substrate: | 0.311 |
| CYP3A4-inhibitor: | 0.595 | CYP3A4-substrate: | 0.343 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.02 | Half-life (T1/2): | 0.032 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.683 | Human Hepatotoxicity (H-HT): | 0.308 |
| Drug-inuced Liver Injury (DILI): | 0.902 | AMES Toxicity: | 0.004 |
| Rat Oral Acute Toxicity: | 0.003 | Maximum Recommended Daily Dose: | 0.286 |
| Skin Sensitization: | 0.976 | Carcinogencity: | 0.207 |
| Eye Corrosion: | 0.772 | Eye Irritation: | 0.654 |
| Respiratory Toxicity: | 0.847 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001647 | 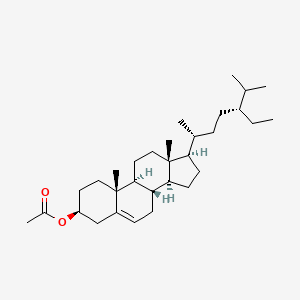 |
0.825 | D0Y7LD | 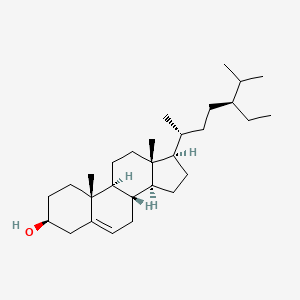 |
0.657 | ||
| ENC000125 | 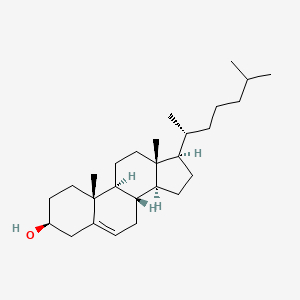 |
0.783 | D0B4RU | 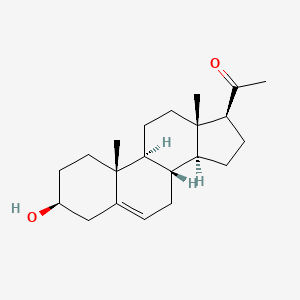 |
0.531 | ||
| ENC003369 | 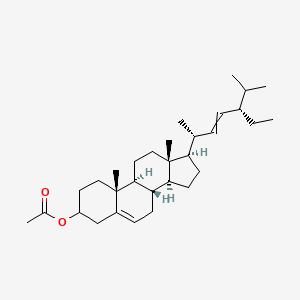 |
0.702 | D06CNP | 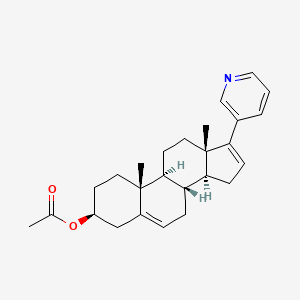 |
0.457 | ||
| ENC001846 | 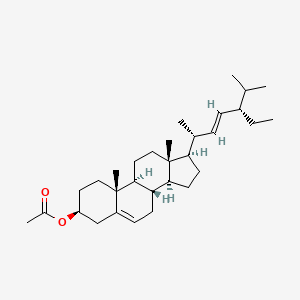 |
0.702 | D0K0EK | 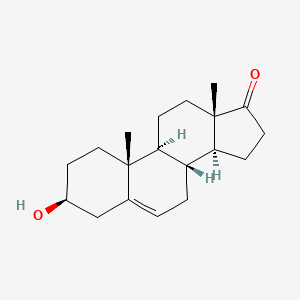 |
0.408 | ||
| ENC000961 | 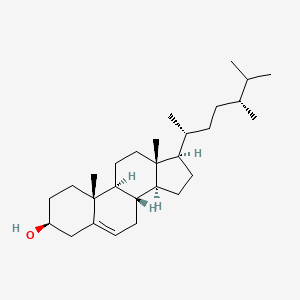 |
0.660 | D07BSQ | 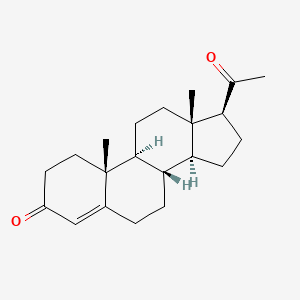 |
0.402 | ||
| ENC001008 | 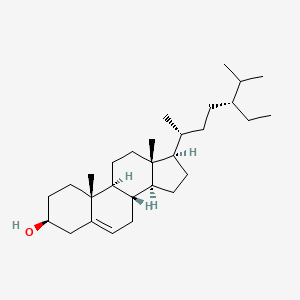 |
0.657 | D02CJX | 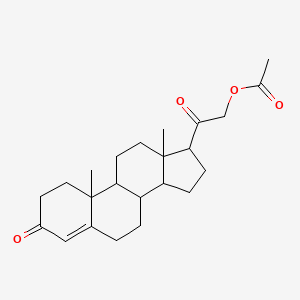 |
0.400 | ||
| ENC001107 | 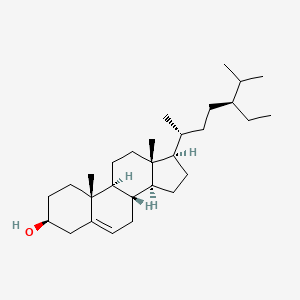 |
0.657 | D03ZTE | 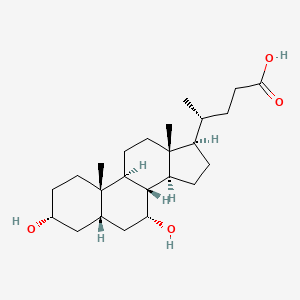 |
0.393 | ||
| ENC001942 | 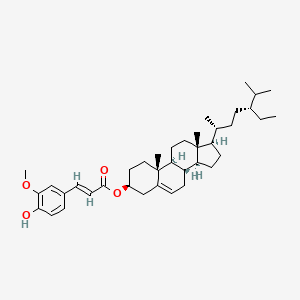 |
0.585 | D0G3SH | 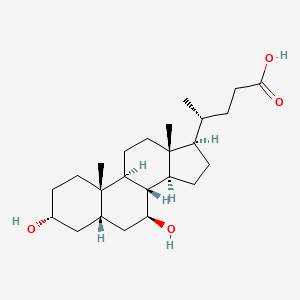 |
0.393 | ||
| ENC005068 | 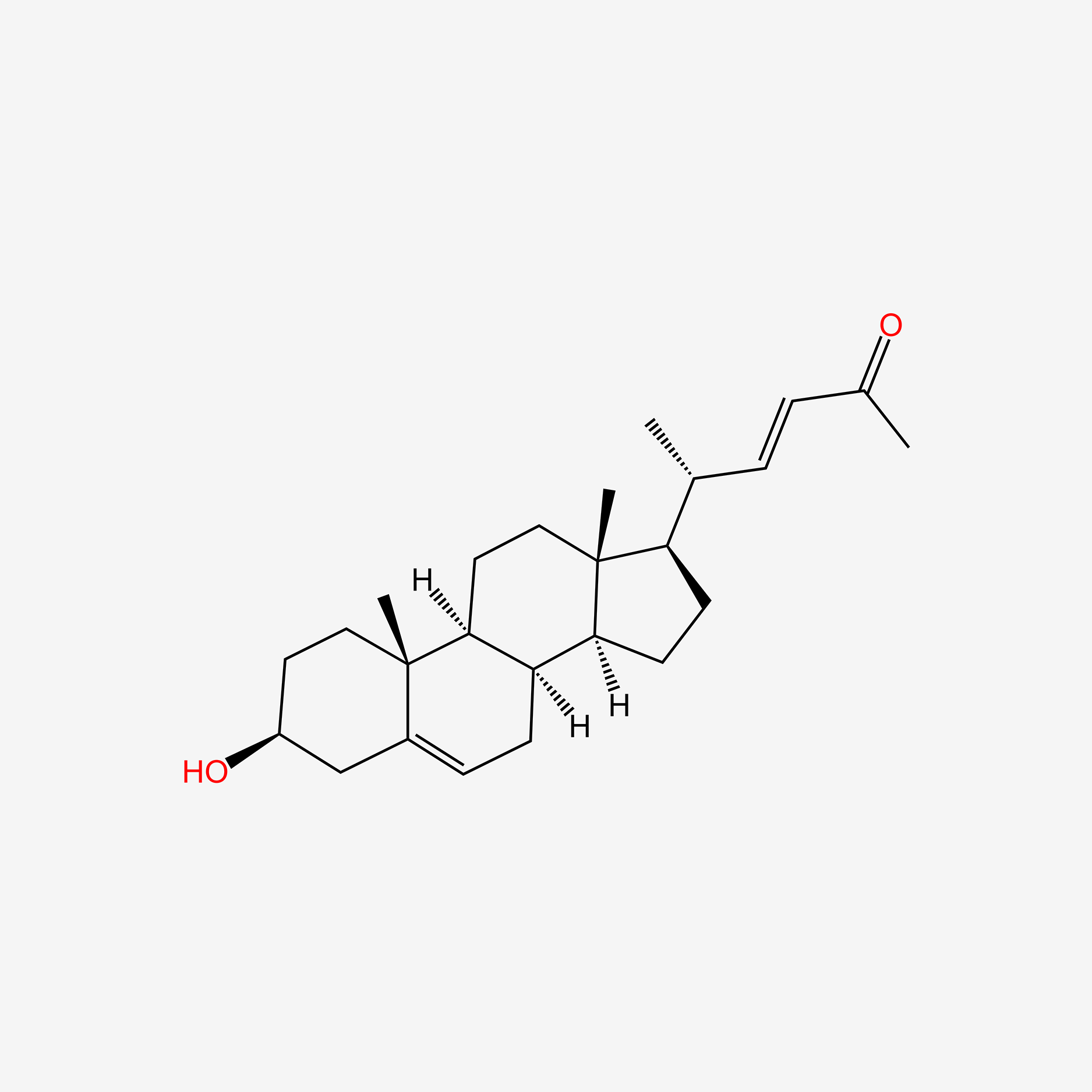 |
0.563 | D0M4WA | 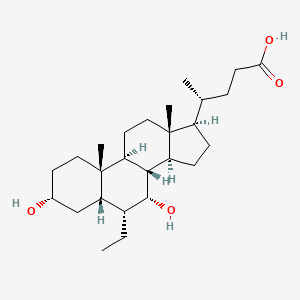 |
0.388 | ||
| ENC001769 | 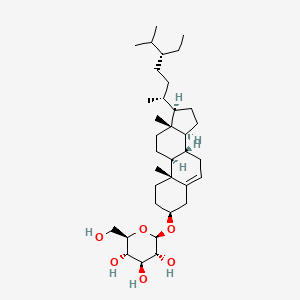 |
0.555 | D0K5WS |  |
0.378 | ||