NPs Basic Information
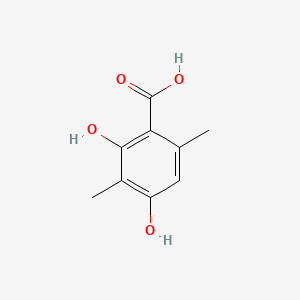
|
Name |
2,4-Dihydroxy-3,6-dimethylbenzoic acid
|
| Molecular Formula | C9H10O4 | |
| IUPAC Name* |
2,4-dihydroxy-3,6-dimethylbenzoic acid
|
|
| SMILES |
CC1=CC(=C(C(=C1C(=O)O)O)C)O
|
|
| InChI |
InChI=1S/C9H10O4/c1-4-3-6(10)5(2)8(11)7(4)9(12)13/h3,10-11H,1-2H3,(H,12,13)
|
|
| InChIKey |
VHNLJRRECIZZPX-UHFFFAOYSA-N
|
|
| Synonyms |
2,4-dihydroxy-3,6-dimethylbenzoic acid; 4707-46-4; 3-METHYLORSELLINIC ACID; 3-Methyl orsellinic Acid; 2,4-DIHYDROXY-3,6-DIMETHYLBENZOICACID; KBio1_001999; 3,6-Dimethyl-2,4-dihydroxybenzoic acid; Spectrum_000611; b-Orcinolcarboxylic acid; SpecPlus_000959; Spectrum2_000413; Spectrum3_000186; Spectrum4_001506; Spectrum5_000271; beta-orcinolcarboxylic acid; Methylorsellinic acid, 3-; BSPBio_001711; KBioGR_002171; KBioSS_001091; SPECTRUM200640; DivK1c_007055; SCHEMBL963740; SPBio_000366; CHEMBL3039269; KBio2_001091; KBio2_003659; KBio2_006227; KBio3_001211; DTXSID20352972; CHEBI:144202; CHEBI:146309; ZINC156441; CCG-38679; MFCD00239340; STK018710; AKOS005378395; SDCCGMLS-0066387.P001; NCGC00095485-01; NCGC00095485-02; BS-17323; DB-070773; 3,6-dimethyl-2,4-bis(oxidanyl)benzoic acid; FT-0614761; D83817; 707D464; A827148; SR-05000002416; 2,4-Dihydroxy-3,6-dimethylbenzoic acid, AldrichCPR; SR-05000002416-1
|
|
| CAS | 4707-46-4 | |
| PubChem CID | 736228 | |
| ChEMBL ID | CHEMBL3039269 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 182.17 | ALogp: | 2.0 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 77.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.62 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.309 | MDCK Permeability: | 0.00000468 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.02 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.066 |
| 30% Bioavailability (F30%): | 0.041 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.104 | Plasma Protein Binding (PPB): | 92.86% |
| Volume Distribution (VD): | 0.403 | Fu: | 6.01% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.087 | CYP1A2-substrate: | 0.455 |
| CYP2C19-inhibitor: | 0.032 | CYP2C19-substrate: | 0.054 |
| CYP2C9-inhibitor: | 0.101 | CYP2C9-substrate: | 0.109 |
| CYP2D6-inhibitor: | 0.034 | CYP2D6-substrate: | 0.136 |
| CYP3A4-inhibitor: | 0.035 | CYP3A4-substrate: | 0.068 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.365 | Half-life (T1/2): | 0.914 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.512 |
| Drug-inuced Liver Injury (DILI): | 0.848 | AMES Toxicity: | 0.029 |
| Rat Oral Acute Toxicity: | 0.27 | Maximum Recommended Daily Dose: | 0.041 |
| Skin Sensitization: | 0.479 | Carcinogencity: | 0.082 |
| Eye Corrosion: | 0.015 | Eye Irritation: | 0.938 |
| Respiratory Toxicity: | 0.821 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005230 | 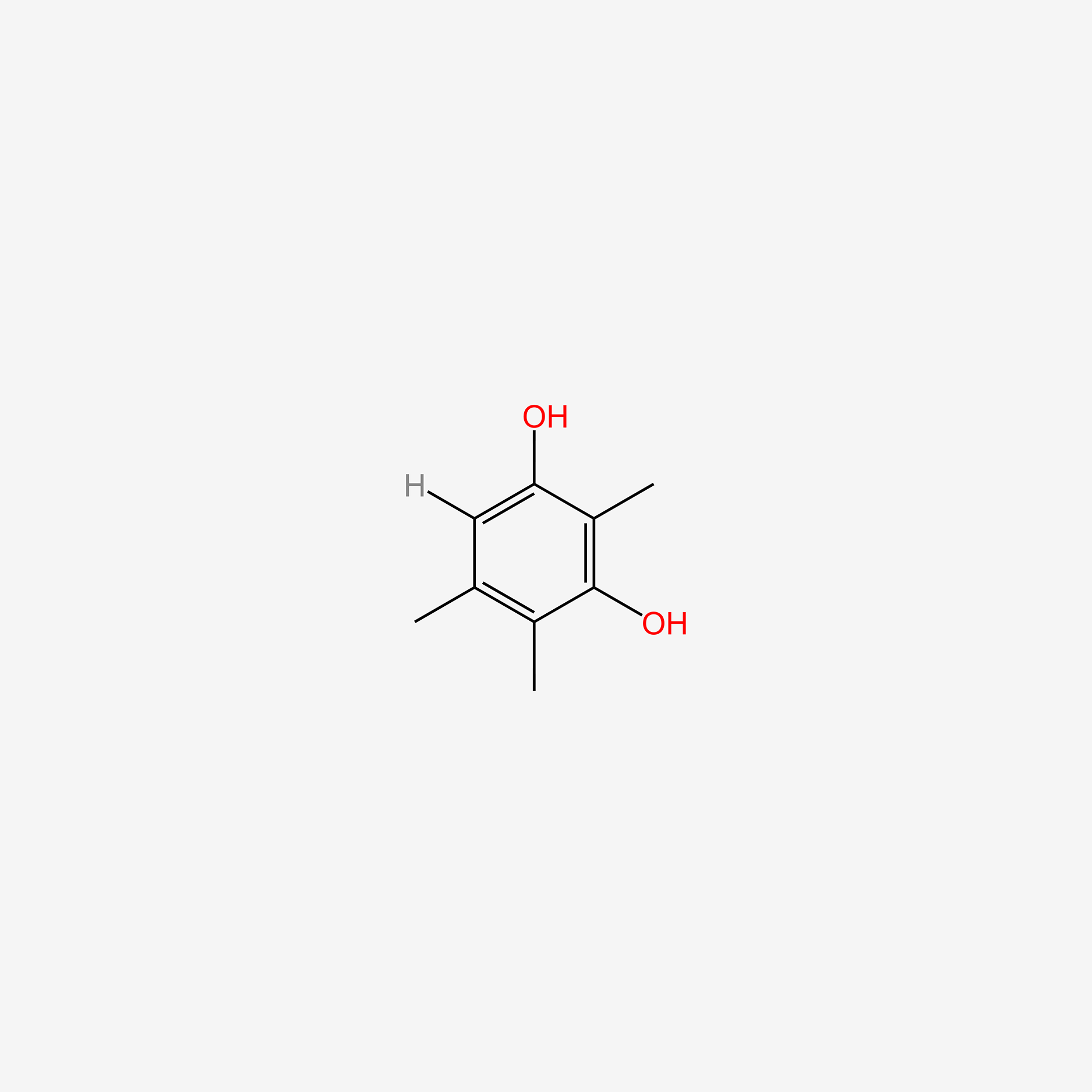 |
0.564 | D0C4YC | 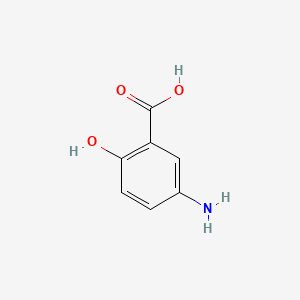 |
0.292 | ||
| ENC002336 | 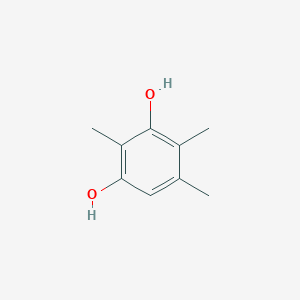 |
0.564 | D01WJL | 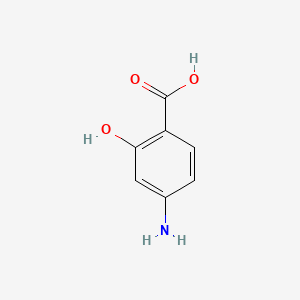 |
0.292 | ||
| ENC000674 | 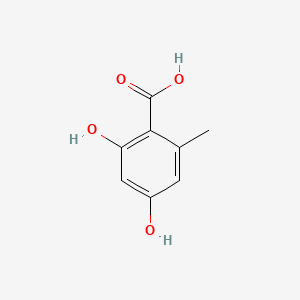 |
0.561 | D0BA6T | 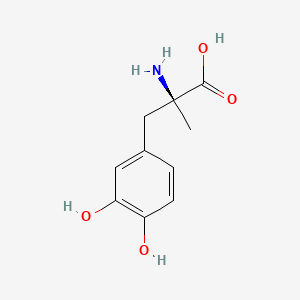 |
0.286 | ||
| ENC001359 | 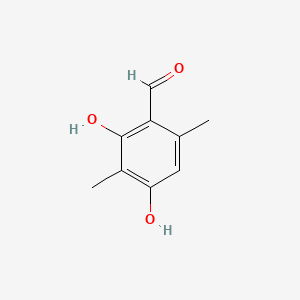 |
0.561 | D0V9EN |  |
0.283 | ||
| ENC002391 | 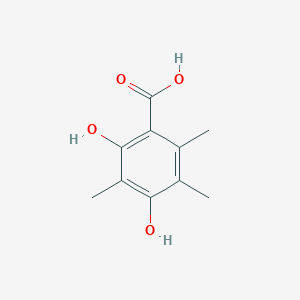 |
0.545 | D05FTJ | 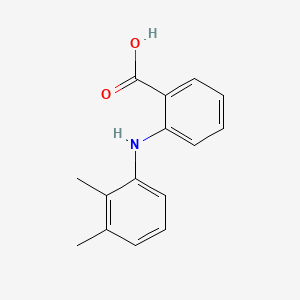 |
0.277 | ||
| ENC001360 | 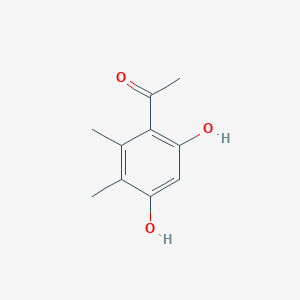 |
0.500 | D08HVR | 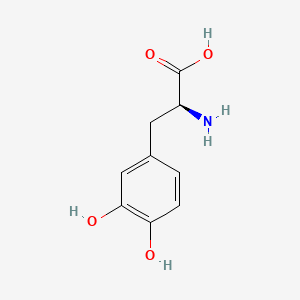 |
0.273 | ||
| ENC003724 | 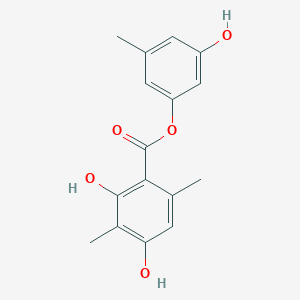 |
0.483 | D0P7JZ | 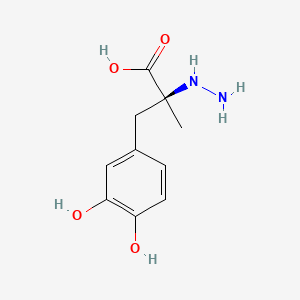 |
0.271 | ||
| ENC001498 | 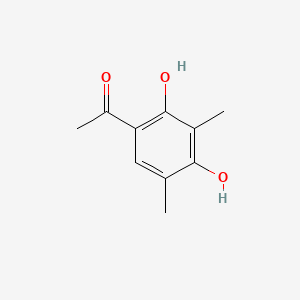 |
0.467 | D0I3RO | 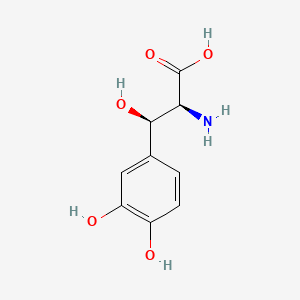 |
0.263 | ||
| ENC005101 | 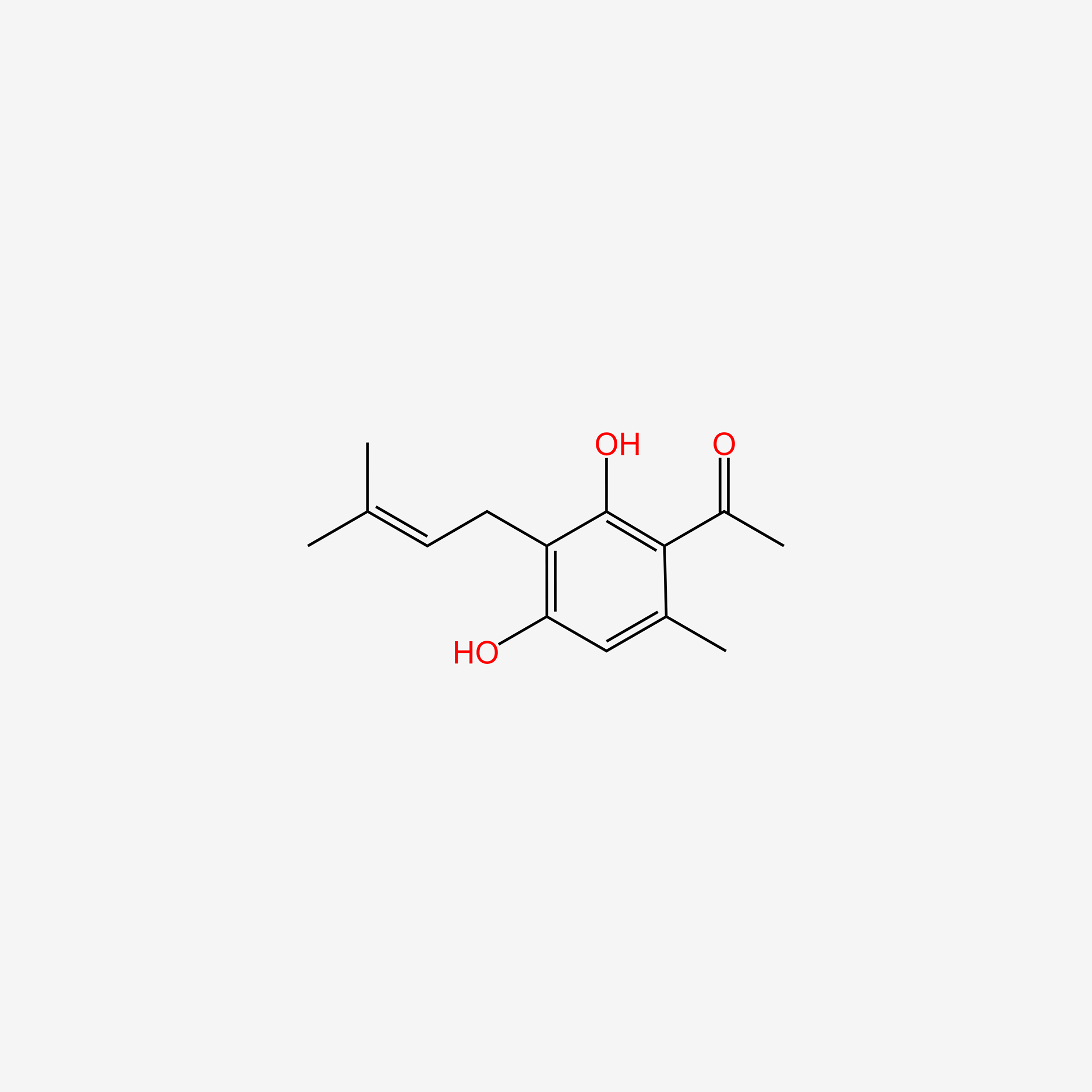 |
0.426 | D0Y7PG | 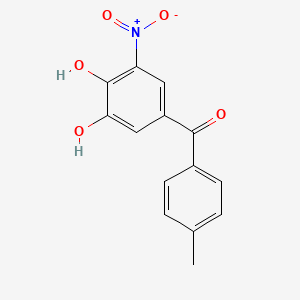 |
0.261 | ||
| ENC005102 | 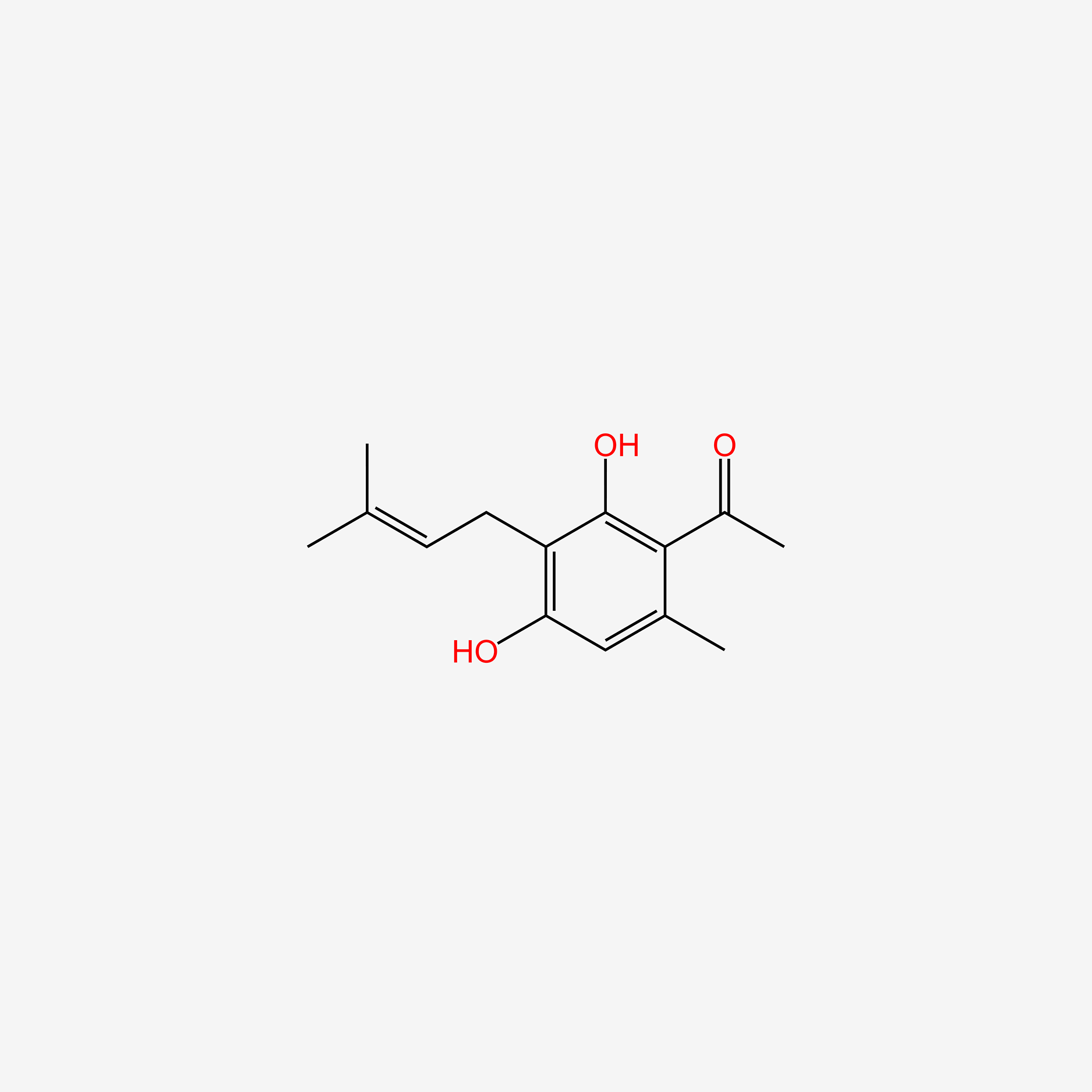 |
0.426 | D07HBX |  |
0.250 | ||