NPs Basic Information
|
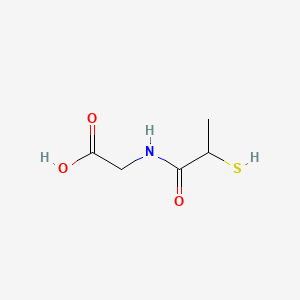
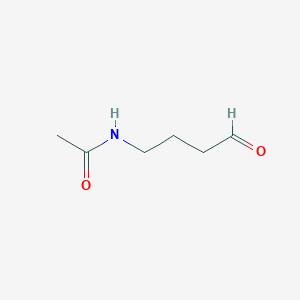
Name |
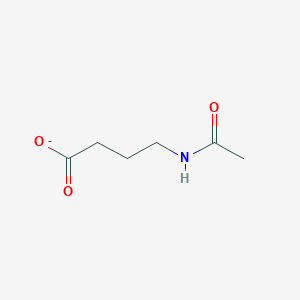
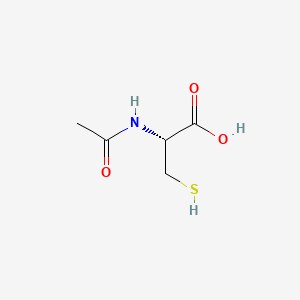
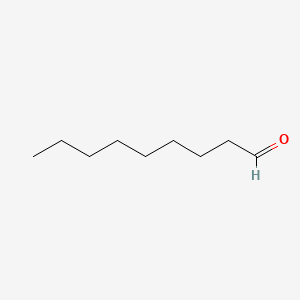
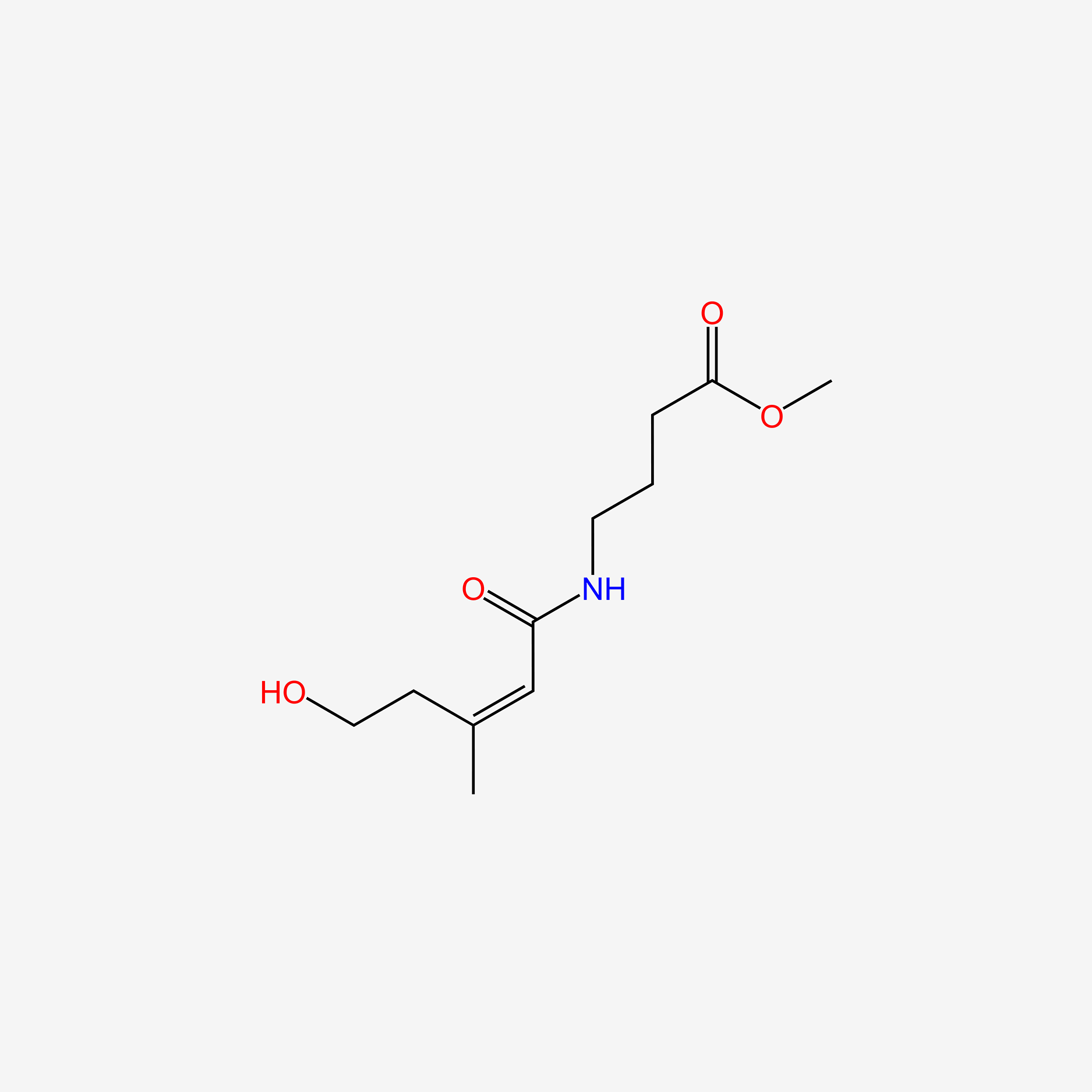
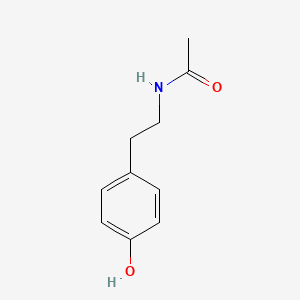
4-Acetamidobutanal
|
| Molecular Formula | C6H11NO2 | |
| IUPAC Name* |
N-(4-oxobutyl)acetamide
|
|
| SMILES |
CC(=O)NCCCC=O
|
|
| InChI |
InChI=1S/C6H11NO2/c1-6(9)7-4-2-3-5-8/h5H,2-4H2,1H3,(H,7,9)
|
|
| InChIKey |
DDSLGZOYEPKPSJ-UHFFFAOYSA-N
|
|
| Synonyms |
4-acetamidobutanal; N-(4-oxobutyl)acetamide; N4-Acetylaminobutanal; 24431-54-7; N-acetyl-4-aminobutanal; 4-(acetylamino)butanal; 4-Acetamidobutyraldehyde; C05936; SCHEMBL293574; CHEBI:7386; N-acetyl-gamma-aminobutyraldehyde; DTXSID30331531; AKOS006348119; EN300-7233403; Q27107484
|
|
| CAS | 24431-54-7 | |
| PubChem CID | 440850 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 129.16 | ALogp: | -0.7 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 9 | QED Weighted: | 0.444 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.44 | MDCK Permeability: | 0.00002140 |
| Pgp-inhibitor: | 0.008 | Pgp-substrate: | 0.108 |
| Human Intestinal Absorption (HIA): | 0.023 | 20% Bioavailability (F20%): | 0.175 |
| 30% Bioavailability (F30%): | 0.283 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.928 | Plasma Protein Binding (PPB): | 22.83% |
| Volume Distribution (VD): | 1.181 | Fu: | 86.49% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.023 | CYP1A2-substrate: | 0.109 |
| CYP2C19-inhibitor: | 0.029 | CYP2C19-substrate: | 0.336 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.106 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.219 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.131 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.285 | Half-life (T1/2): | 0.745 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.06 |
| Drug-inuced Liver Injury (DILI): | 0.033 | AMES Toxicity: | 0.775 |
| Rat Oral Acute Toxicity: | 0.04 | Maximum Recommended Daily Dose: | 0.04 |
| Skin Sensitization: | 0.852 | Carcinogencity: | 0.012 |
| Eye Corrosion: | 0.022 | Eye Irritation: | 0.533 |
| Respiratory Toxicity: | 0.155 |