NPs Basic Information
|
Name |
Antimycin A3
|
| Molecular Formula | C26H36N2O9 | |
| IUPAC Name* |
[(2R,3S,6S,7R,8R)-8-butyl-3-[(3-formamido-2-hydroxybenzoyl)amino]-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate
|
|
| SMILES |
CCCC[C@@H]1[C@H]([C@@H](OC(=O)[C@H]([C@H](OC1=O)C)NC(=O)C2=C(C(=CC=C2)NC=O)O)C)OC(=O)CC(C)C
|
|
| InChI |
InChI=1S/C26H36N2O9/c1-6-7-9-18-23(37-20(30)12-14(2)3)16(5)36-26(34)21(15(4)35-25(18)33)28-24(32)17-10-8-11-19(22(17)31)27-13-29/h8,10-11,13-16,18,21,23,31H,6-7,9,12H2,1-5H3,(H,27,29)(H,28,32)/t15-,16+,18-,21+,23+/m1/s1
|
|
| InChIKey |
PVEVXUMVNWSNIG-PDPGNHKXSA-N
|
|
| Synonyms |
Antimycin A3; Blastomycin; Blastmycin; Antimycin A3b; 522-70-3; Purothionin AII; NSC58239; 97YBD5W80B; CHEMBL436605; 116095-17-1; A 80021F34; A3; (2R-(2R*,3S*,6S*,7R*,8R*))-8-Butyl-3-(3-formamidosalicylamido)-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl isovalerate; [(2R,3S,6S,7R,8R)-3-[(3-Formamido-2-hydroxybenzoyl)amino]-8-heptyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] (2R)-2-methylbutanoate; [(2R,3S,6S,7R,8R)-8-butyl-3-[(3-formamido-2-hydroxybenzoyl)amino]-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate; UNII-97YBD5W80B; A1-Purothionin; ANTIMYCIN A3B [MI]; SCHEMBL158729; DTXSID7037189; CHEBI:175924; ZINC4212164; BDBM50107132; NSC-58239; NCI60_004417; Q27272047; WLN: T9OV EOVTJ CMVR CQ DMVH& D1 G4 HOVX1&1&1 I1; Threonine, 2-(1,2-dihydroxypropyl)hexanoate, 7-lactone, isovalerate; (2R,3S,6S,7R,8R)-8-butyl-3-(3-formamido-2-hydroxybenzamido)-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl 3-methylbutanoate; (2R,3S,6S,7R,8R)-8-butyl-3-[(3-formamido-2-hydroxybenzene)amido]-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl 3-methylbutanoate; [(2R,3S,6S,7R,8R)-8-butyl-3-[(3-formamido-2-hydroxy-benzoyl)amino]-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate; [(2R,3S,6S,7R,8R)-8-butyl-3-[(3-ormamido-2-hydroxybenzoyl)amino]-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate; 3-Methyl-butyric acid (2R,3S,6S,7R,8R)-8-butyl-3-(3-formylamino-2-hydroxy-benzoylamino)-2,6-dimethyl-4,9-dioxo-[1,5]dioxonan-7-yl ester; 3-Methyl-butyric acid (2R,3S,6S,7R,8R)-8-butyl-3-{[1-(3-formylamino-2-hydroxy-phenyl)-methanoyl]-amino}-2,6-dimethyl-4,9-dioxo-[1,5]dioxonan-7-yl ester; 3-Methyl-butyric acid 8-butyl-3-(3-formylamino-2-hydroxy-benzoylamino)-2,6-dimethyl-4,9-dioxo-[1,5]dioxonan-7-yl ester; BUTANOIC ACID, 3-METHYL-, (2R,3S,6S,7R,8R)-8-BUTYL-3-((3-(FORMYLAMINO)-2-HYDROXYBENZOYL)AMINO)-2,6-DIMETHYL-9-OXO-1,5-DIOXONAN-7-YL ESTER; Butanoic acid, 3-methyl-, 8-butyl-3-((3-(formylamino)-2-hydroxybenzoyl)amino)-2,6-dimethyl-9-oxo-1,5-dioxonan-7-yl ester, (2R-(2R*,3S*,6S*,7R*,8R*))-; Isovaleric acid,9-dimethyl-2,6-dioxo-1,5-dioxonan-3-yl)-3-formamidosalicylamide, stereoisomer
|
|
| CAS | 116095-17-1 | |
| PubChem CID | 245869 | |
| ChEMBL ID | CHEMBL436605 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 520.6 | ALogp: | 4.2 |
| HBD: | 3 | HBA: | 9 |
| Rotatable Bonds: | 10 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 157.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 37 | QED Weighted: | 0.182 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.842 | MDCK Permeability: | 0.00003990 |
| Pgp-inhibitor: | 0.403 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.985 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.942 | Plasma Protein Binding (PPB): | 81.11% |
| Volume Distribution (VD): | 0.574 | Fu: | 14.07% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.146 | CYP1A2-substrate: | 0.064 |
| CYP2C19-inhibitor: | 0.371 | CYP2C19-substrate: | 0.089 |
| CYP2C9-inhibitor: | 0.77 | CYP2C9-substrate: | 0.944 |
| CYP2D6-inhibitor: | 0.148 | CYP2D6-substrate: | 0.139 |
| CYP3A4-inhibitor: | 0.883 | CYP3A4-substrate: | 0.15 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.206 | Half-life (T1/2): | 0.478 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.776 |
| Drug-inuced Liver Injury (DILI): | 0.893 | AMES Toxicity: | 0.012 |
| Rat Oral Acute Toxicity: | 0.019 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.071 | Carcinogencity: | 0.172 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.016 |
| Respiratory Toxicity: | 0.014 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
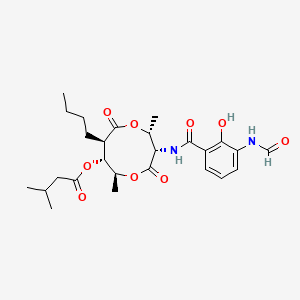
| ENC000483 | 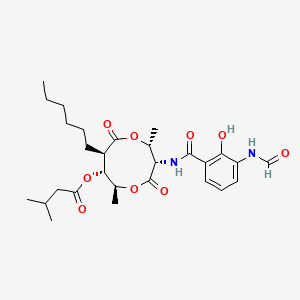 |
0.925 | D00OAY | 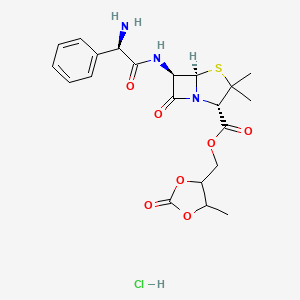 |
0.260 | ||
| ENC001503 | 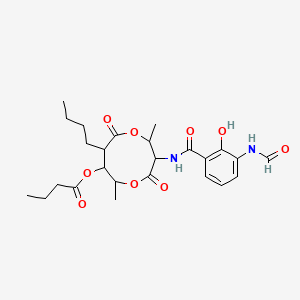 |
0.850 | D07IPB |  |
0.259 | ||
| ENC001502 |  |
0.789 | D0T5XN |  |
0.254 | ||
| ENC000878 |  |
0.284 | D06TQZ |  |
0.241 | ||
| ENC000586 | 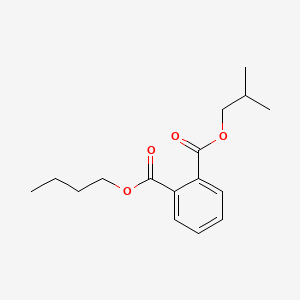 |
0.260 | D0HD9K | 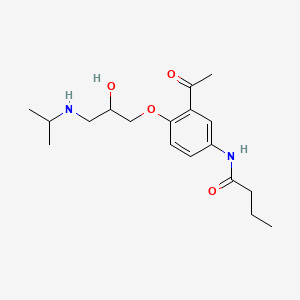 |
0.241 | ||
| ENC004061 | 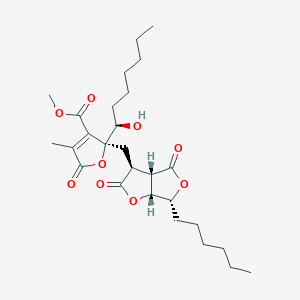 |
0.259 | D0A0JH | 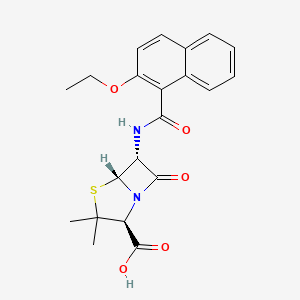 |
0.233 | ||
| ENC004760 | 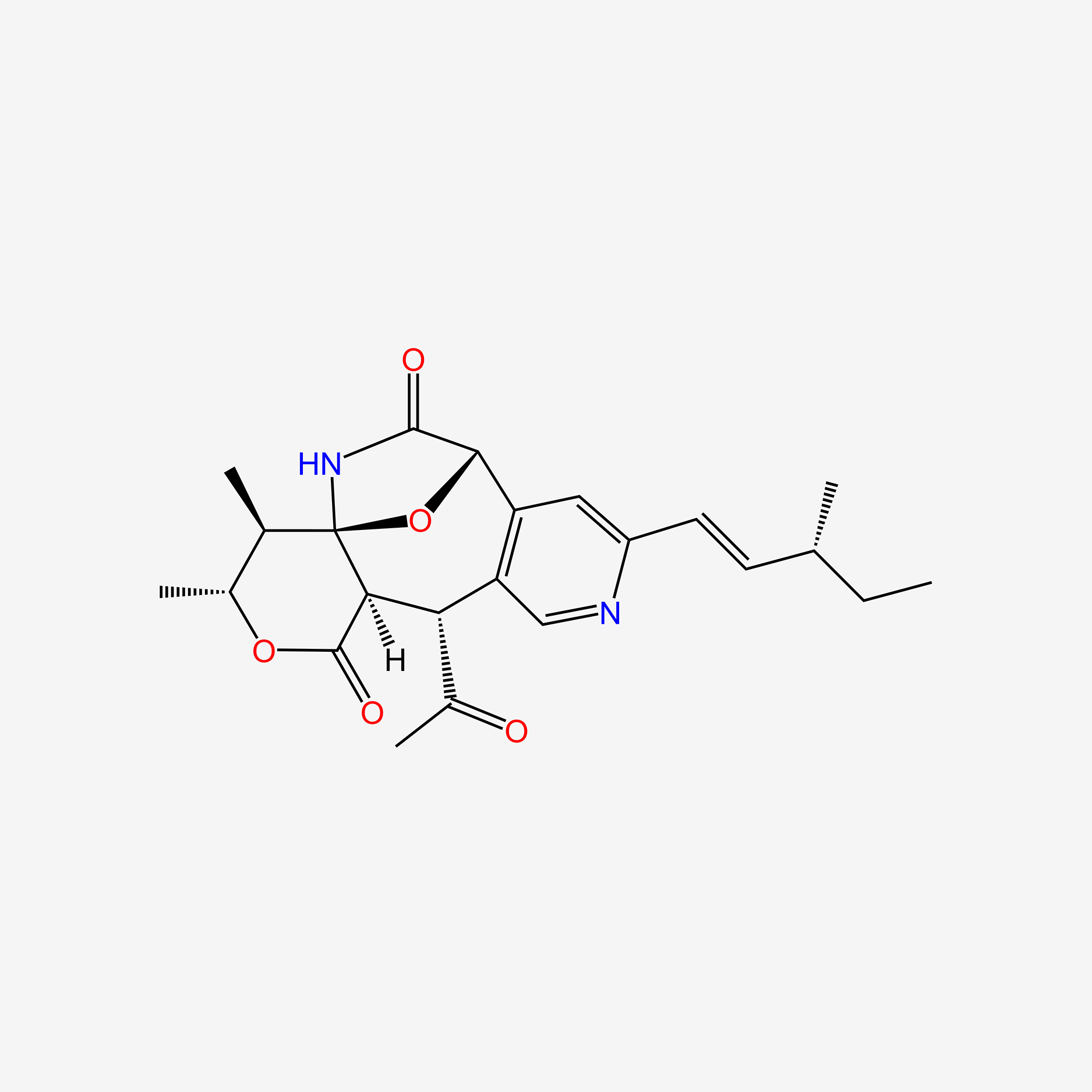 |
0.255 | D0T9TJ | 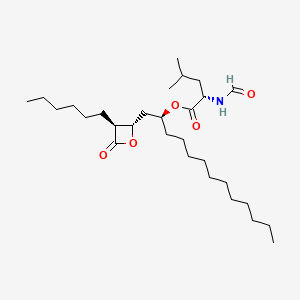 |
0.230 | ||
| ENC003393 | 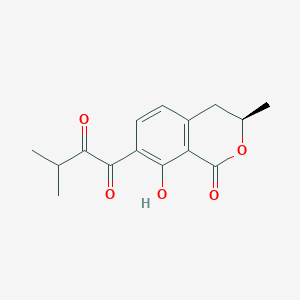 |
0.254 | D0X1WJ | 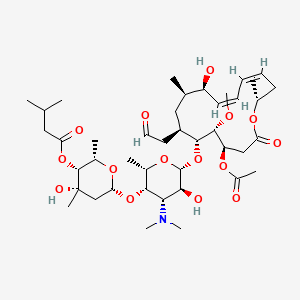 |
0.225 | ||
| ENC001802 | 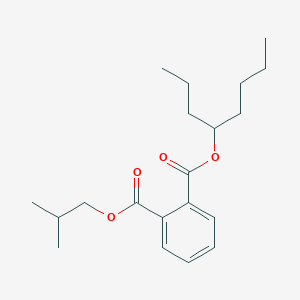 |
0.248 | D06WTZ | 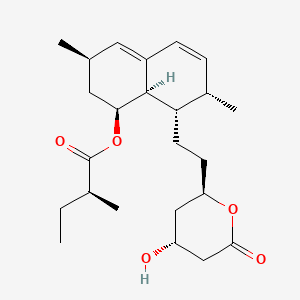 |
0.224 | ||
| ENC003926 | 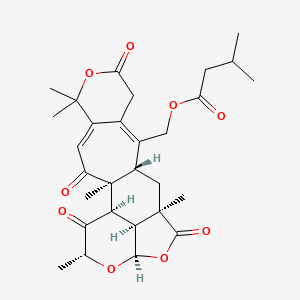 |
0.247 | D0R2KJ | 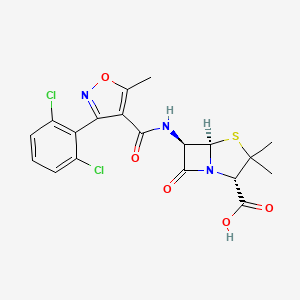 |
0.223 | ||