NPs Basic Information
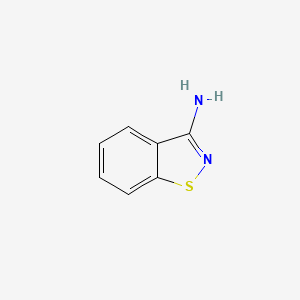
|
Name |
1,2-Benzisothiazol-3-amine
|
| Molecular Formula | C7H6N2S | |
| IUPAC Name* |
1,2-benzothiazol-3-amine
|
|
| SMILES |
C1=CC=C2C(=C1)C(=NS2)N
|
|
| InChI |
InChI=1S/C7H6N2S/c8-7-5-3-1-2-4-6(5)10-9-7/h1-4H,(H2,8,9)
|
|
| InChIKey |
WIJQCPIRWXSWQG-UHFFFAOYSA-N
|
|
| Synonyms |
1,2-Benzisothiazol-3-amine; 23031-78-9; 1,2-benzothiazol-3-amine; benzo[d]isothiazol-3-amine; 3-Amino-1,2-benzisothiazole; MLS000081875; SMR000060378; 613262-33-2; aminobenzoisothiazole; EINECS 245-388-4; 3-aminobenzisothiazole; 3-amino-benzisothiazole; 3-iminobenzisothiazoline; 1,2-benzothiazol-3-ylamine; benzo[d]isothiazol-3-ylamine; cid_89966; SCHEMBL290849; 3-aminobenzo(2,1)isothiazole; CHEMBL1458786; SCHEMBL11654916; BDBM38954; DTXSID40177588; HMS1753B15; HMS2490B20; ZINC854665; AMY28840; MFCD00726295; AKOS001012886; AC-5045; CS-W019021; FS-2862; DB-025529; FT-0646047; EN300-188513; 031A789; A816516; AA-516/30012037; J-503796; Z56774438
|
|
| CAS | 23031-78-9 | |
| PubChem CID | 89966 | |
| ChEMBL ID | CHEMBL1458786 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 150.2 | ALogp: | 2.0 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 67.2 | Aromatic Rings: | 2 |
| Heavy Atoms: | 10 | QED Weighted: | 0.626 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.813 | MDCK Permeability: | 0.00003920 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.019 |
| Human Intestinal Absorption (HIA): | 0.021 | 20% Bioavailability (F20%): | 0.066 |
| 30% Bioavailability (F30%): | 0.561 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.997 | Plasma Protein Binding (PPB): | 95.84% |
| Volume Distribution (VD): | 1.164 | Fu: | 8.09% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.865 | CYP1A2-substrate: | 0.894 |
| CYP2C19-inhibitor: | 0.256 | CYP2C19-substrate: | 0.47 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.495 |
| CYP2D6-inhibitor: | 0.962 | CYP2D6-substrate: | 0.884 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.174 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.289 | Half-life (T1/2): | 0.757 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.046 | Human Hepatotoxicity (H-HT): | 0.963 |
| Drug-inuced Liver Injury (DILI): | 0.756 | AMES Toxicity: | 0.823 |
| Rat Oral Acute Toxicity: | 0.923 | Maximum Recommended Daily Dose: | 0.255 |
| Skin Sensitization: | 0.824 | Carcinogencity: | 0.878 |
| Eye Corrosion: | 0.898 | Eye Irritation: | 0.987 |
| Respiratory Toxicity: | 0.967 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000177 | 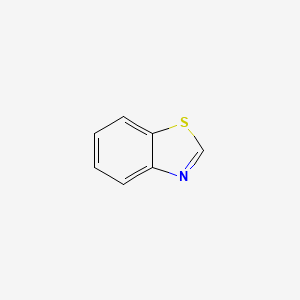 |
0.400 | D0K1XK | 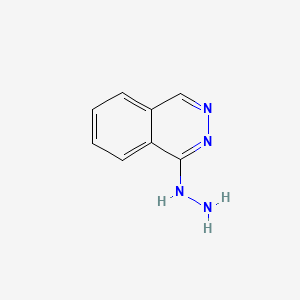 |
0.362 | ||
| ENC000675 | 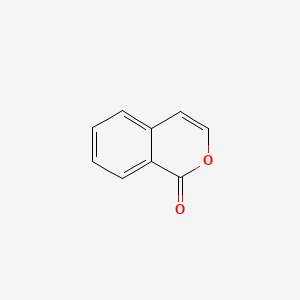 |
0.326 | D09ZIS | 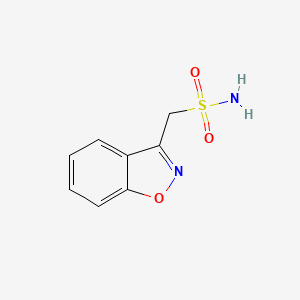 |
0.333 | ||
| ENC000167 | 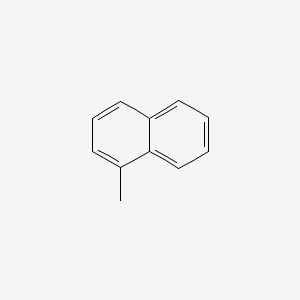 |
0.326 | D0E6YQ | 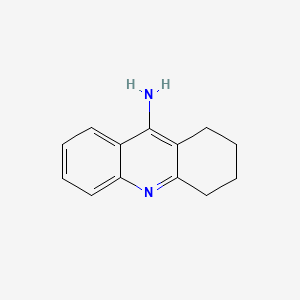 |
0.327 | ||
| ENC000341 |  |
0.326 | D0QS1U | 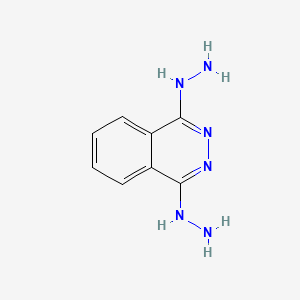 |
0.327 | ||
| ENC005757 | 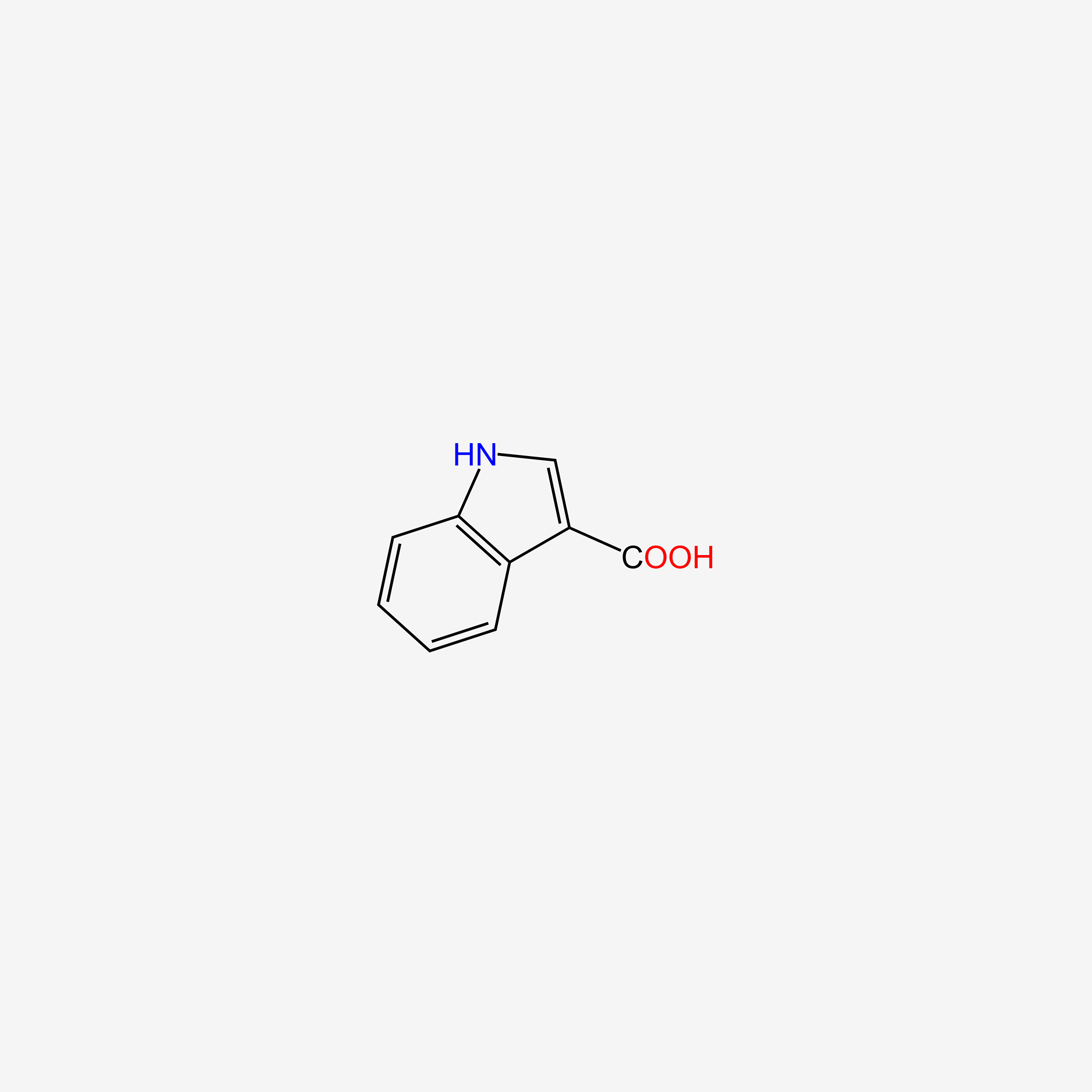 |
0.313 | D0D5GG | 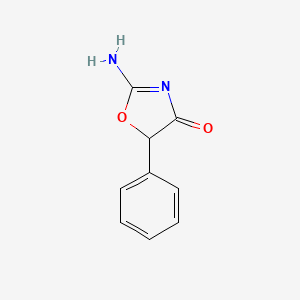 |
0.320 | ||
| ENC002806 | 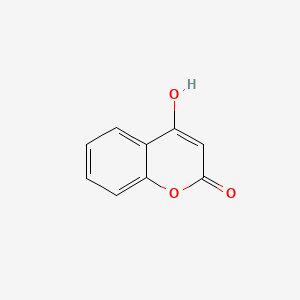 |
0.313 | D06CTE | 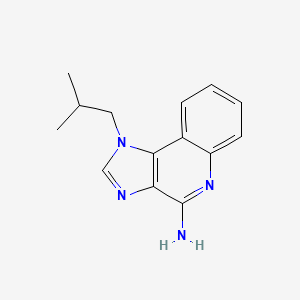 |
0.311 | ||
| ENC000303 | 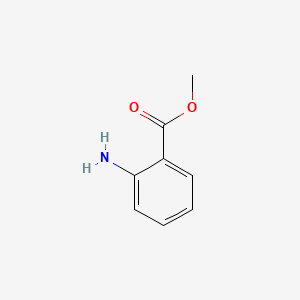 |
0.311 | D09JUG | 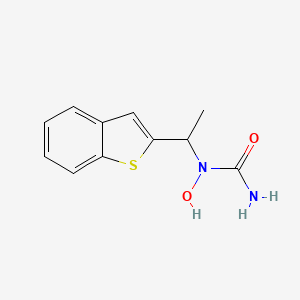 |
0.304 | ||
| ENC001333 |  |
0.311 | D00VUL | 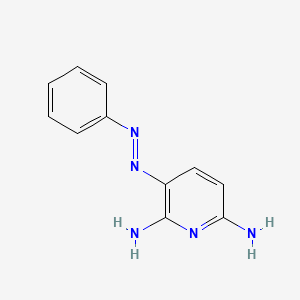 |
0.293 | ||
| ENC001799 | 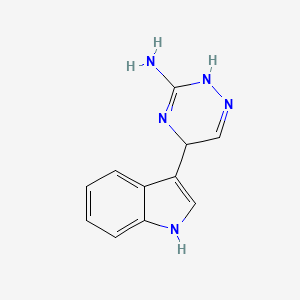 |
0.310 | D05EJG | 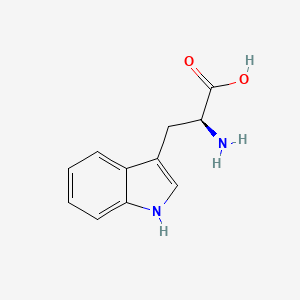 |
0.291 | ||
| ENC001448 | 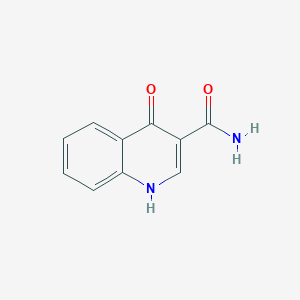 |
0.308 | D07HBX |  |
0.273 | ||